More Information
Submitted: October 16, 2023, 2023 | Approved: October 21, 2024 | Published: October 22, 2024
How to cite this article: German KE, Rumyantsev AS, Manukova VA. Research of Potential Production 94mTc in Medical Cyclotron. J Clin Intensive Care Med. 2024; 9(1): 027-030. Available from: https://dx.doi.org/10.29328/journal.jcicm.1001050
DOI: 10.29328/journal.jcicm.1001050
Copyright license: © 2024 German KE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: 94mTc; PET imaging; Medical cyclotron; Liquid target
Research of Potential Production 94mTc in Medical Cyclotron
German KE1*, Rumyantsev AS1,2 and Manukova VA2
1Frumkin Institute of Physical Chemistry and Electrochemistry RAS, Moscow, Russia
2A.N. Bakulev Scientific Center of Cardiovascular Surgery, Moscow, Russia
*Address for Correspondence: German KE, Frumkin Institute of Physical Chemistry and Electrochemistry RAS, Russia, Moscow, Email: guerman_k@mail.ru
To expand the spectrum of used radiopharmaceuticals, it is proposed to obtain a positron-emitting isotope of technetium 94mTc. The intention of this work is to research the possibility of producing various technetium isotopes on a medical cyclotron. For this purpose, we carried out a series of irradiations of an aqueous solution of molybdenum of natural isotopic composition with protons of 11 MeV energy. After technetium isolation, results were analyzed on a γ-spectrometer. 511 keV gamma-ray line was obtained.
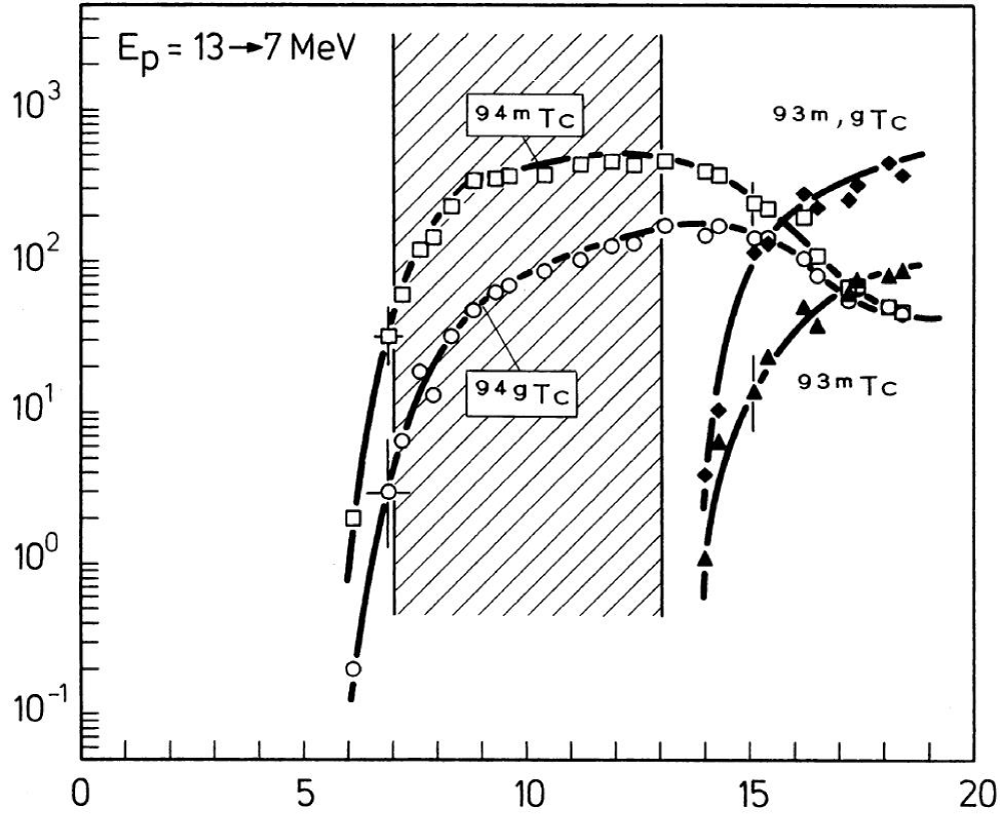
Positron emission tomography combined with computed tomography (PET/CT) is a highly informative diagnostic method in various areas of medicine, such as oncology, and cardiology, as well as in the diagnosis of inflammatory processes. In this regard, it is important to introduce new positron radionuclides for the synthesis of radiopharmaceuticals (RP), which will have a number of advantages over other RP labeled with widely used radionuclides 18F, 11C, and 13N. One of the promising positrons (β+) emitters is the technetium isotope 94mTc, which has optimal nuclear-physical characteristics for use in PET, such as a positron branching ratio of 72% and a half-life of 52 min, allowing the synthesis of RP. The broad potential of 94mTc is due to the possibility of using existing commercial kits designed for the manufacture of RP from generator 99mTc-pertechnetate [1]. For example, 94mTc-sestamibi can be used as a myocardial perfusion agent [2]. Scientists from the USA conducted comparative research on eight patients with a history of myocardial infarction with radiopharmaceuticals 13N-ammonia and 94mTc-sestamibi [3] . The authors did not reveal significant differences in the accumulation of RPs in the heart muscle, but the administered dose of 94mTc-sestamibi is lower than that of 13N-ammonia. In the same way, the technetium isotope 94mTc can be used for a wide range of radiopharmaceuticals as a radioactive label instead of 99mTc to obtain PET images. There are many methods to obtain 94mTc. However, the most suitable method for practical work is to irradiate a stable isotope of molybdenum 94Мо with protons. The reaction 94Mo(p,n)94mTc gives out a high yield of the target radionuclide and a low content of isotopic impurity 94gTc about 6% - 10% [4]. In addition, 94gTc has a short half-life of 293 min and is itself a partial positron emitter (positron branching ratio 11%), so it will not create excess radiation exposure to patients. However, this method works with proton energy from 8 to 13 MeV only. Scientists from Germany conducted a study and found that above 13 MeV, the probability of a nuclear reaction with releases two neutrons from 94Mo and the formation of isotopes 93g,mTc begins to increase rapidly, which is shown in the graph in Figure 1 [5]. Accordingly, the production of 94mTc by the nuclear reaction 94Mo(p,n)94mTc is possible only at low-energy accelerators, such as the medical cyclotron Siemens RDS-111 with a proton energy of 11 MeV. It also takes into consideration what is this cyclotron, as well as most other medical cyclotrons, designed for irradiation of liquid and gas target substances only. That’s why molybdenum must be used in the form of an aqueous solution. In addition, there are methods for relatively rapid extraction of technetium from the liquid phase [6,7].
Figure 1: Excitation functions of 94Mo(p,xn)-processes leading to the formation of 94m,gTc and 93m,gTc. (Qaim_Syed_M,_2000).
The essence of the experiment was to produce positron-emitting 94mTc using the capabilities of a small radiochemical laboratory at the PET center. To achieve this, we faced 4 tasks:
a) Selection of the composition of a molybdenum solution for irradiation on a cyclotron;
b) Selection and programming of the target device;
c) Development of a method for purifying Tc from positron-emitting isotopes in an irradiated solution;
d) Definition of qualitative analysis of the obtained product for the technetium isotope composition.
- Preparation of liquid target solution
In this research, it is irradiated molybdenum of natural isotopic composition (Table 1).
| Table 1: Natural isotopic composition of molybdenum. | ||||||
| 92Mo | 94Mo | 95Mo | 96Mo | 97Mo | 98Mo | 100Mo |
| 14,6% | 9,2% | 15,9% | 16,7% | 9,6% | 24,3% | 9,7% |
As a target substance was used, an aqueous solution of potassium molybdate with a molybdenum concentration of about 100 mg/ml, was obtained by dissolving dry molybdenum (VI) oxide in 1М КОН.
- Cyclotron targets
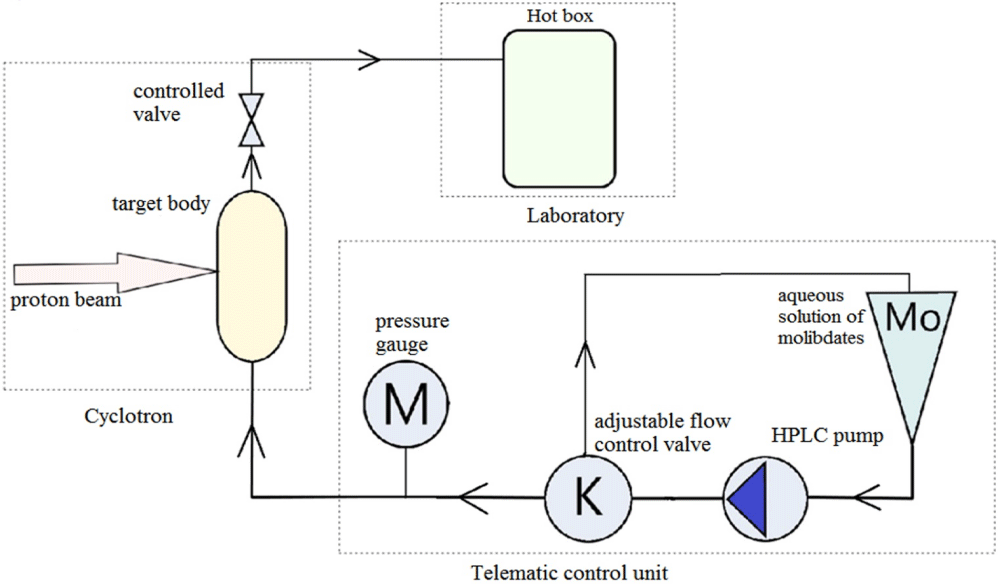
Work on the production of technetium was carried out on the Siemens RDS-111 cyclotron. The target device is a modified standard TCU designed to produce radionuclide nitrogen-13. This allows the entire cycle of obtaining radionuclides to be carried out automatically. The operating principle of this device is shown schematically in Figure 2. The HPLC pump with a capacity of 1.5 ml/min feeds a solution of the target substance into the volume 3 ml aluminum target through a mechanical valve set to a system pressure of 300 PSI and the pump constantly supports it during irradiation. After irradiation, a remotely controlled valve opens and the pump flows over the target solution into a hot box in the laboratory.
Figure 2: Layout of the target and transfer system for Tc production.
- Purification of technetium isotopes from radionuclide impurities
The produced technetium is stabilized in the chemical form of an aqueous solution of pertechnetate. The main part of radionuclide impurities to technetium are positron-emitting radionuclides, which are obtained from water by reactions 18O(p;n)18F (Т1/2=110 min) and 16O(p;α)13N (Т1/2=10 min). For this research, the obtained solution is delayed until 13N is fully decayed for at least 2 hours. 18F is formed as fluoride anions and is removed from the solution by precipitation with CaF2 as an isotopic carrier:
Ca2+ + 19F- + 18F- = Ca19F18F↓; (Ca2+ + TcO4- ≠).
- Radioisotope measurements by γ-spectroscopy
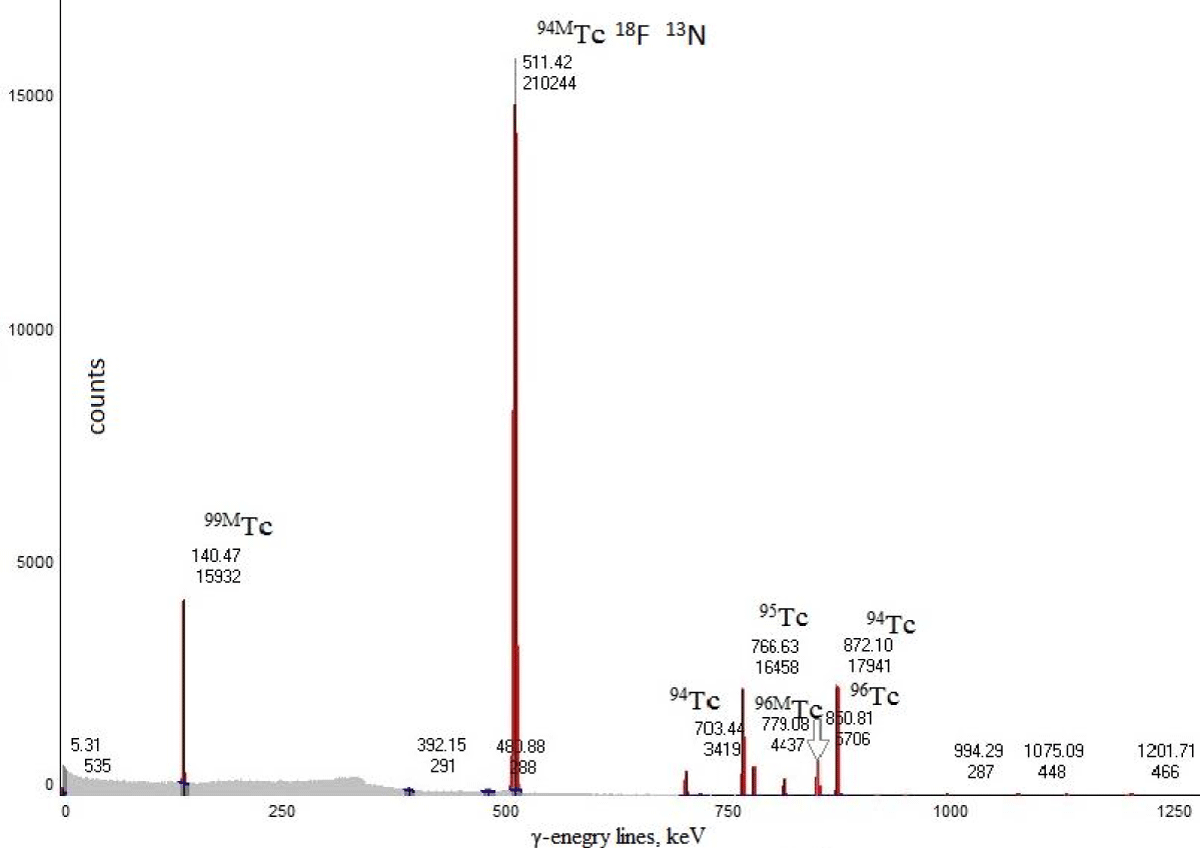
A 100 µl sample with an activity of about 1 MBq was added to a 3 ml vial of water and mixed thoroughly. The radionuclide composition was assessed using a γ-spectrometer HPGe with detector GEM30, manufacturer ORTEC. Since positrons annihilate with the emission of γ-quantum with energy 511 keV, then along this line it is possible to identify positron emitters including 94mTc, which does not have its own unique γ-line. As can be seen from Table 2, other isotopes of technetium have unique γ-energy lines. Shows itself especially well 99mТc, having a single, separate from other isotopes γ-line 140,45 keV.
| Table 2: γ-energy lines (keV) used for identification of technetium isotopes. | ||||||||||
| 99mТc | 95Tc | β+ | 94gTc | 95Tc | 96mТc | 96gТc | 94gТc | 94m,gTc | 95Tc | 95Tc |
| 140,45 | 204,14 | 511,4 | 703,3 | 766,6 | 779,06 | 813,4 | 850,7 | 872,1 | 948,8 | 1075 |
Isotopes technetium production
In the work, several irradiations with a current of 30 μA were performed from 5 to 30 minutes. The pressure in the target during irradiation was constantly maintained at 300 PSI. Nuclear reactions with molybdenum isotopes can generate the radionuclides, presented in Table 3.
| Table 3: Nuclear reactions for the production of the different Tc isotopes and their specification [8,9]. | ||
| Reaction | Half-life | Decay |
| 94Mo(p,n)94gТc | 293 min | 87,9% EC; 11,1% β+ |
| 94Mo(p,n)94mТc | 52 min | 29,8% EC; 72,2% β+ |
| 95Mo(p,n)5Tc | 1200 min | 100% EC |
| 96Mo(p,n)96gТc | 6163 min | 100% EC |
| 96Mo(p,n)96mТc | 52 min | 98% IT; 2% IC |
| 100Mo(p,2n)99mТc | 360 min | 88% IT; 12% IC |
Accordingly, if we use isotopically pure Mo, we will obtain the only isotope we need, for example, 94mTc from 94Mo. This will also ensure high yields of the target radionuclide from irradiation. The only difficulty is the high cost of isotopically pure molybdenum. And since the physical consumption of molybdenum for the production of technetium is very low, it is possible to regenerate molybdenum for its repeated irradiation.
Pertechnetate isolation
2 ml of 0.1 M NaF was pre-added to the vial prepared for collecting the target substance. After unloading the irradiated solution and mixing, 0.5 M CaCl2 was added dropwise to the solution until a white precipitate completely formed. It was then passed through a sterilizing syringe nozzle with 0.22 µm pores, 18F together with precipitate was retained on the filter, and TcO4- passed into the filtrate. More effective 18F-fluoride removal is also possible, if the CaCl2 solution is placed in advance, and the isotopic carrier NaF is added after unloading the target substance.
Results of γ-spectroscopy
After cleaning from 18F and a delay of more than 2 hours for decay of 13N, the samples were analyzed on an γ-spectrometer. Spectrum was obtained and deciphered in the form of a diagram in Figure 3. There is a clean line at 140 keV, belonging to the isotope 99mТc, and line 511 кэВ, belonging to positron emitters. As well as lines belonging to other isotopes of technetium. It turns out that this method can be used for the production of 99mTc from stable 100Mo, which has a great advantage, compared to technetium from the generator, in the absence of the long-lived parent radionuclide 99Mo [10].
Figure 3: Spectrum of the sample with decoding.
Dynamic γ-spectroscopy by the line 511 keV
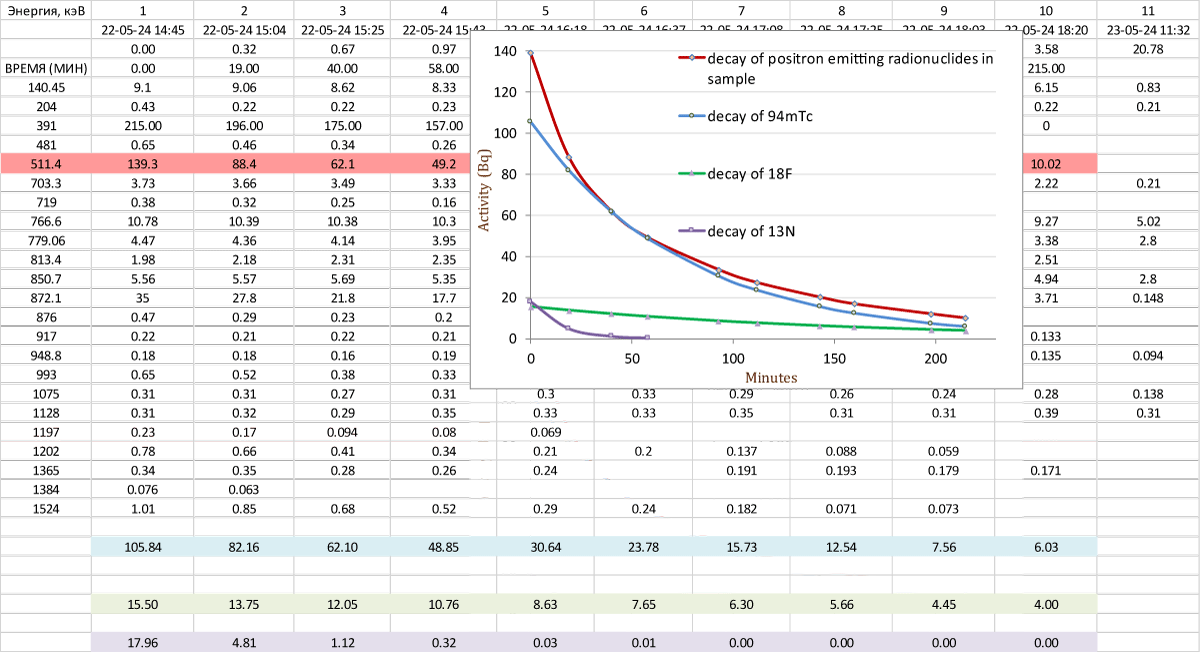
One of the samples was analyzed on an γ-spectrometer every 30 minutes. Based on the obtained data was done graphic representation of the decay rate of positron emitters in the sample (Figure 4). A comparative analysis was carried out with the decay rate of 94mТc, taking the coinciding points at 30 and 60 minutes as the calculated activity. As can be seen from the graphic, the decay of the sample almost coincides with the decay of 94mТc, this indirectly confirms that among positron emitters 94mТc gives the largest share to the line 511 keV. Additionally, a graphic was done of the decay rate of 18F, taking as the calculated value activity the difference between «decay of positron-emitting r/n» and «decay of 94mТc» in the last point of the graph. The decay rate of 13N is taken as the calculated value activity of the difference between decay graphics in the first point of the graph. As can be seen, these graphics are far from the main one, and therefore their content is at the of small impurities.
Figure 4: Comparison of decays +¦+-emitting radionuclides at a frequency of 511 keV.
For further scientific work in this direction, it is necessary first of all refine the separation methodology pertechnetate anions not only from fluoride but also from 13N in anionic form. After which, to synthesize the radiopharmaceuticals, it will be necessary to construct a new or redesign already existing automated radiochemistry synthesizer. The synthesis itself is hypothetically ordinary – useful RPs, labeled with the isotope 99mТc, and methods of their analysis are used everywhere. To obtain 94mТc without other isotopes of technetium expensive isotopically pure 94Mo is required. And the main problem is allocation with minimal losses of molybdenum from irradiated solution. Without this, the research direction will not be economically justified.
This paper demonstrates the possibility of obtaining various technetium isotopes in a liquid target device of a low-energy proton accelerator. Particular attention should be paid to the positron-emitting isotope 94mTc. The optimal half-life of 52 minutes allows chemistry reactions with 94mTc to obtain radiopharmaceuticals in PET diagnostics. In the future, if the irradiation parameters are optimized and isotopically pure 94Mo is used in the target substance, it will be possible to obtain high yields of the target radionuclide.
- Kovács Z. Technetium-99m pharmaceuticals: preparation and quality control in nuclear medicine. Berlin: Springer; 2006:151-3.
- Szajek LP, Der M, Dive J, Huang BX, Plascjak P, Eckelman WC. Production and radioassay of Tc-94m for PET studies. Radiochim Acta. 2003;91:613-6. Available from: https://doi.org/10.1002/jlcr.25804401269
- Stone CK, Christian BT. Technetium 94m-labeled methoxyisobutyl isonitrile: dosimetry and resting cardiac imaging with positron emission tomography. J Nucl Cardiol. 1994;1(5):425-33. Available from: https://doi.org/10.1007/bf02961596
- Qaim SM. Production of high purity 94mTc for positron emission tomography studies. Nucl Med Biol. 2000;27:323-8. Available from: https://doi.org/10.1016/s0969-8051(00)00104-9
- Rosch F, Qaim SM. Nuclear data relevant to the production of the positron emitting technetium isotope 94mTc via the 94Mo(p,n)-reaction. Radiochim Acta. 1993;62:115-121. Available from: https://doi.org/10.1524/ract.1993.62.3.115
- Hoehr C, Morley T, Buckley K, Trinczek M, Hanemaayer V, Schaffer P, et al. Radiometals from liquid targets: 94mTc production using a standard water target on a 13 MeV cyclotron. Appl Radiat Isot. 2012;70:2308-12. Available from: https://doi.org/10.1016/j.apradiso.2012.06.004
- Harper R, Morim DR, Mehta D, Rosecker V, Archibald SJ, Southworth R, et al. Optimised production of technetium-94m for PET imaging by proton-irradiation of phosphomolybdic acid in cyclotron liquid target. Appl Radiat Isot. 2024;210:111381. Available from: https://doi.org/10.1016/j.apradiso.2024.111381
- Lamere E, Couder M, Beard M, Simon A, Simonetti A, Skulski M, et al. Proton-induced reactions on molybdenum. Phys Rev C. 2019;100:034614. Available from: https://doi.org/10.1103/PhysRevC.100.034614
- Sun H, Han R, Liu B, Chen Z, Yang B, Zhang X, et al. Thick target yields of proton-induced reactions on natural molybdenum. Appl Radiat Isot. 2022;190:110474. Available from: https://doi.org/10.1016/j.apradiso.2022.110474
- Martini P, Boschi A, Cicoria G, Corazza A, Zagni F, Uccelli L, et al. In-house cyclotron production of high-purity Tc-99m and Tc-99m radiopharmaceuticals. Appl Radiat Isot. 2018;139:325-31. Available from: https://doi.org/10.1016/j.apradiso.2018.05.033