More Information
Submitted: July 14, 2023 | Approved: July 10, 2024 | Published: July 11, 2024
How to cite this article: Bassami M, Taghipour B, Eslami R, Hoseinpour AN, Dawkins K, Singar S, et al. The Effects of Interval and Traditional Resistance Exercise on Hormonal Control of Adipose-tissue Lipolysis in Healthy Young Men. J Clin Intensive Care Med. 2024; 9: 021-026. Available from: https://dx.doi.org/10.29328/journal.jcicm.1001049.
DOI: 10.29328/journal.jcicm.1001049
Copyright license: © 2024 Bassami M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Lipolysis; Hormonal control; Fat oxidation; Interval resistance exercise; Norepinephrine; Epinephrine
Abbreviations: GH: Growth Hormone; Camp: Controlling Intracellular Cyclic AMP; PKA: Protein Kinase A; FFAs: Free Fatty Acids; TRT: Traditional Resistance Training; HIIT: High-Intensity Interval Resistance Training
The Effects of Interval and Traditional Resistance Exercise on Hormonal Control of Adipose-tissue Lipolysis in Healthy Young Men
Minoo Bassami1,2* , Bahareh Taghipour1, Rasul Eslami1, Ali Nejatian Hoseinpour1, Kallie Dawkins3, Saiful Singar3 and Bahram H Arjmandi3*
, Bahareh Taghipour1, Rasul Eslami1, Ali Nejatian Hoseinpour1, Kallie Dawkins3, Saiful Singar3 and Bahram H Arjmandi3*
1Department of Exercise Physiology, Faculty of Physical Education and Sports Sciences, Allameh Tabataba’i University, Tehran, Iran
2Research institutes of Sport Science and Health, Allameh Tabataba’i University, Tehran, Iran
3Professor, Florida State University, Tallahassee, FL, USA
*Address for Correspondence: Dr. Bahram H Arjmandi, Professor, Florida State University, 120 Convocation Way, Tallahassee, FL, USA, Email: Barjmandi@fsu.edu
Dr. Minoo Bassami, Department of Exercise Physiology, Faculty of Physical Education and Sports Sciences, Allameh Tabataba’i University, Opposite to Olympic Hotel, 9th Chougan Street, Tehran, Iran, Email: bassami@atu.ac.ir
Purpose: Lipolysis is regulated by lipolytic hormones, like insulin, cortisol, growth Hormone (GH), and catecholamines. Unregulated lipolysis results in the accumulation of free fatty acids (FFAs), leading to dysfunction of cells and death. Thus, the main aim of this study was to determine the effects of interval and traditional resistance exercise on hormonal control of adipose-tissue lipolysis in healthy young men.
Methods: Twelve healthy males (Mean ± SD; age, 25.5 ± 3.1 years; Body mass index, 24.2 ± 2.0 kg/m2) performed tradition resistance exercise (TRE) at 80% of 1RM (3 sets of 6 repetitions) with 2 min passive recovery, and an interval resistance exercise (IRE) trial at 60% of 1RM (3 sets of 6 repetitions) followed by active recovery (1 set of 6 repetitions at 20% of 1RM). Three blood samples were taken before and immediately after exercise, and after one-hour recovery and were analyzed to measure epinephrine, norepinephrine, cortisol, and GH.
Results: Statistical analyses of the data revealed that concentrations of cortisol and GH increased in response to resistance exercise and significantly decreased (p < 0.05) during the recovery period. Although there were no significant differences between the two protocols for cortisol concentration, GH increases following IRE were profoundly higher than TRE protocol. Epinephrine and norepinephrine increased (p < 0.05) in response to both resistance exercise trials, though, no between-group differences were found for these variables.
Conclusion: The results of our study showed increases in GH, cortisol, epinephrine, and norepinephrine in two resistance exercise protocols which may lead to increases in fat oxidation.
In recent years, there has been a notable surge in interest within the realms of health, fitness, and lifestyle towards the exploration of effective exercise modalities aimed at optimizing metabolic outcomes. Adipose tissue lipolysis assumes a pivotal role in energy metabolism, with its regulation intricately guided by hormonal signals [1-3]. Lipolytic hormones, such as insulin, cortisol, Growth Hormone (GH), and catecholamines can influence lipolysis by controlling intracellular cyclic AMP (cAMP) and the activity of Protein Kinase A (PKA) [4-10]. Epinephrine and norepinephrine have the most important regulatory role in adipose tissue during metabolism and modulate lipolysis with increasing cAMP concentration and PKA activity [11,12]. FFAs accumulate in peripheral metabolic tissues, such as liver, muscle, and pancreatic islets due to unregulated lipolysis, and may lead to cell dysfunction and death [13].
The majority of research has studied the effects of aerobic exercise, finding that resistance exercise may improve overall body composition, and change lipid metabolism [14-16].
Furthermore, it has been noted that resistance training promotes the breakdown of fat cells in obese individuals [17,18]. Additionally, engaging in regular physical activities outside of structured exercise has been found to have a beneficial effect on the body’s composition and resting metabolic rate among older adults, as it increases muscle mass and daily energy expenditure while reducing fat mass [18,19]. The number of exercises, intensity, and number of repetitions, intervals, and sets are the parameters of a resistance training program, which may lead to different responses of regulatory hormones [20]. Epinephrine and norepinephrine increased significantly after three equal consecutive cycles of resistance training; a protocol designed to stress the major muscle groups and was performed by untrained young men [21]. Raymond, et al. [22] showed that resistance exercise may increase cortisol concentration with higher intensity. One study measured GH secretion for 12 hours after an acute heavy resistance exercise and demonstrated that resistance exercise elevated GH levels for 20 minutes after exercise immediately in young males [23]. Yet, several studies showed a greater GH response in both high repetition with 70% or more, and high total work with short intervals [24].
However, manipulating the intensity of an exercise, recovery type, and work-to-recovery ratio may lead to vastly different results in resistance exercise adaptations [25,26]. It has been suggested that resistance exercise with shorter rest intervals is more effective and useful for increasing lipolysis and oxidation [27]. A study measuring respiratory exchange ratio and energy consumption reported that 22 hours after Traditional Resistance Training (TRT) and High-Intensity Interval Resistance Training (HIIRT), there was a greater amount of energy expenditure after HIIRT than TRT. This study also demonstrated a decrease in the respiratory exchange ratio, showing that fat utilization was dominant in HIIRT. However, it is important to note that hormonal changes were not investigated [28]. In another study, after performing a 45-minute HIIRE session (including six repetitions with 80% 1RM with 20 seconds rest, three repetitions with 80% 1RM -with 20 seconds rest followed by two repetitions with 80% 1RM - with 20 seconds rest, which is performed twice) and a 65 minutes TRE session (including three sets of exercises with eight repetitions at 75% of 1RM, with 2 ′30″ of rest between the sets), no significant difference was shown between the two protocols. However, it is important to note that participants in this study were overweight young girls and other regulatory hormones were not investigated [29].
Therefore, to our knowledge, no study has focused on lipolytic hormonal responses following both a Traditional Resistance Exercise (TRE) and an Interval Resistance Exercise (IRE) session. Hence, our objective was to examine how these two exercise protocols affect the hormonal control of adipose tissue lipolysis in healthy young men. By investigating the impact of these exercise modalities on hormonal responses associated with adipose tissue lipolysis, our study aims to provide valuable insights for designing targeted and effective exercise strategies to improve metabolic health. The findings of this research have the potential to optimize exercise prescriptions and customize interventions to enhance the hormonal regulation of adipose tissue lipolysis. Ultimately, this research contributes to a deeper understanding of how exercise influences metabolic health in young, healthy males.
Participants
Following approval from the University’s research degree and Ethics Committee (IR.SBU.RCE.1399.003), twelve healthy males were recruited to take part in the study (Table 1). Participants were excluded if they were active smokers or took medications/supplements known to affect substrate metabolism. Participants were required to refrain from consuming caffeine and performing exercise 24 hours before each laboratory visit. All participants were informed both verbally and in writing about the purpose, risks, and benefits of the research, and gave their written informed consent to participate in this investigation. All experimental procedures were performed by the Declaration of Helsinki.
| Table 1: Subjects’ characteristics (mean ± SD). | ||||
| Age (yrs) | Height (cm) | Body Mass (kg) | Body mass index (kg/m2) | Body fat (%) |
| 25.5 ± 3.1 | 174.1.5 ± 7.8 | 73.9 ± 1.0 | 24.2 ± 2.0 | 16.8 ± 4.6 |
Procedures
Determination of maximum strength (1RM): To minimize the risk of unnecessary musculoskeletal injury, all subjects performed a warm-up that consisted of two phases. The first phase was a 5-minute warm-up on a cycle ergometer (Monark ergometric 839E, Sweden) at a self-selected intensity. The participant’s 1RM lifts for the exercises used during the strength testing period were determined using previously described procedures [30].
Experimental procedures: Subjects reported to the laboratory on four separate occasions. The first session was designed to familiarize the subjects with the procedures and to determine their anthropometric characteristics (stature, body mass, and percentage of body fat). The second session was to determine the one-repetition maximum (1RM), with the third and fourth sessions to perform the TRE and IRE protocols. Participants arrived at the laboratory under the same pretesting conditions for each visit (between 7 am - 8 am). Subjects completed a food diary on the day before their first test and repeated this diet before the second trial. Subjects were instructed to refrain from drinking alcohol and to not engage in any strenuous exercise 24 hours before all visits. Two exercise trials were allocated randomly in a counterbalanced manner and separated by at least 7 days.
Subjects reported to the physiology laboratory following an overnight fast, one hour before the main exercise protocol. They were given a light breakfast (two slices of toast with 150 g carrot jam and a 250 ml cup of orange juice), which contained approximately 650 kcal. One hour after consuming breakfast, resting blood pressure was measured (Omron M7, Omron Ltd, Kyoto, Japan), and a blood draw (11 ml) was taken. Like during the 1RM trial, all exercise was preceded by a two-phase warm-up.
The TRE protocol used in the present study was similar to that previously reported [30]. Briefly, it involved conducting 3 sets of 6 repetitions at 80% of 1RM of the six exercises stated previously, with 2 minutes of passive rest. The IRE protocol comprised 3 sets of 6 repetitions at 60% of 1RM with active recovery (one set of 6 repetitions at 20% of 1RM for the same exercises). Two exercise trials were performed in a counterbalance manner in two separate weeks and exercise volume (sets × reps × amount of weight) was kept equal for both protocols. The second and third blood draws were taken immediately after exercise and following one-hour recovery.
Data collection and laboratory methods
Venous blood samples were obtained from an antecubital vein at rest (before exercise), immediately after exercise, and after one-hour recovery. Plasma was obtained by collecting a blood sample into a pre-treated EDTA vacutainer. These samples were gently mixed before centrifugation (4 ºC for 15 minutes at 1900 g). After centrifugation, plasma was separated and stored at –70 ºC for the subsequent analysis of cortisol, epinephrine, norepinephrine, and GH. Catecholamines were analyzed using the Human 2CAT Epinephrin\Norepinephrine Elisa kit (96t-LDN, Germany); cortisol was analyzed by a German ZellBio Cortizol kit, and GH was analyzed by a Human GH Elisa kit (Monobind, Germany). All parameters were assayed by ELISA (Enzyme-linked immunosorbent assay) technique using a fully automated system (DRG instruments Gmbh, Germany).
Statistical analyses
All statistical analyses were performed using the software statistical package SPSS version 22. After confirming the normality of the data by the Shapiro-Wilk Test, a repeated measure of ANOVA (analysis of variance) (2 conditions × 3 times) was employed to evaluate the differences in the mean values of blood parameters between the two exercise protocols. When ANOVA indicated the presence of a significant difference, the Tukey post hoc test was used to identify which mean differences were statistically significant. Values are presented as mean (±SE), unless otherwise stated, with the level of significance set at p ≤ 0.05.
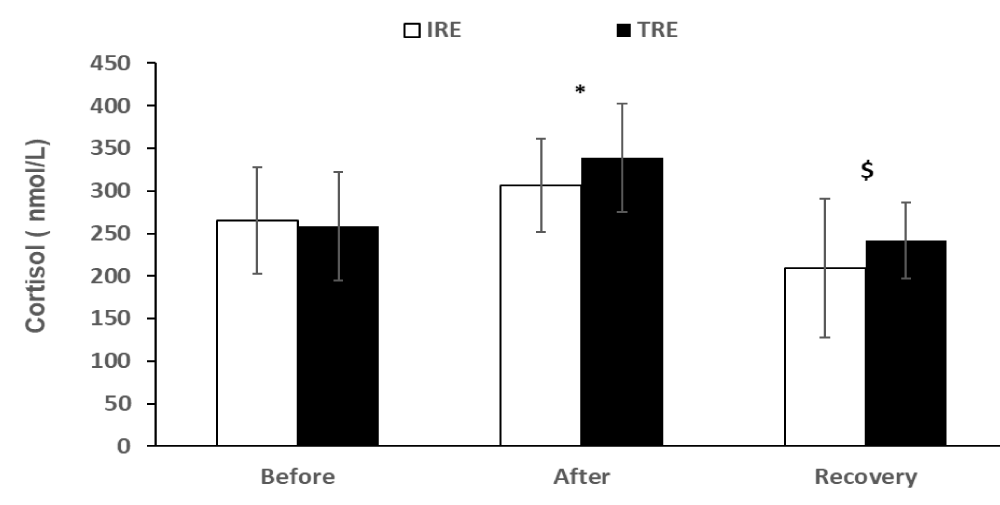
The statistical analyses of the data showed a main significant effect of exercise on cortisol (p < 0.001) and GH (p < 0.001). Post-hoc analyses indicated that concentrations of cortisol and GH were elevated (p < 0.001) after resistance exercise and significantly decreased during the recovery period (Figures 1,2). However, there was no significant difference in cortisol concentration between the two protocols (F2, 22 = 1.7, p > 0.05). On the other hand, the increase in GH following the IRE protocol was significantly higher compared to the TRE protocol (F2, 22 = 8.07, p < 0.01).
Figure 1: Cortisol values (means ± SD) before and after exercise, and after the recovery period for two exercise protocols. *indicates a significant (p < 0.01) exercise effect, and $ represents a significant (p < 0.01) recovery effect. IRE, interval resistance exercise. TRE is a traditional resistance exercise.
Figure 2: Growth hormone values (means ± SD) before and after exercise, and after the recovery period for two exercise protocols. * indicates a significant (p < 0.001) exercise effect, $ represents a significant (p < 0.001) recovery effect, and # indicates a significant (p < 0.01) difference between the protocols. IRE, interval resistance exercise. TRE is a traditional resistance exercise.
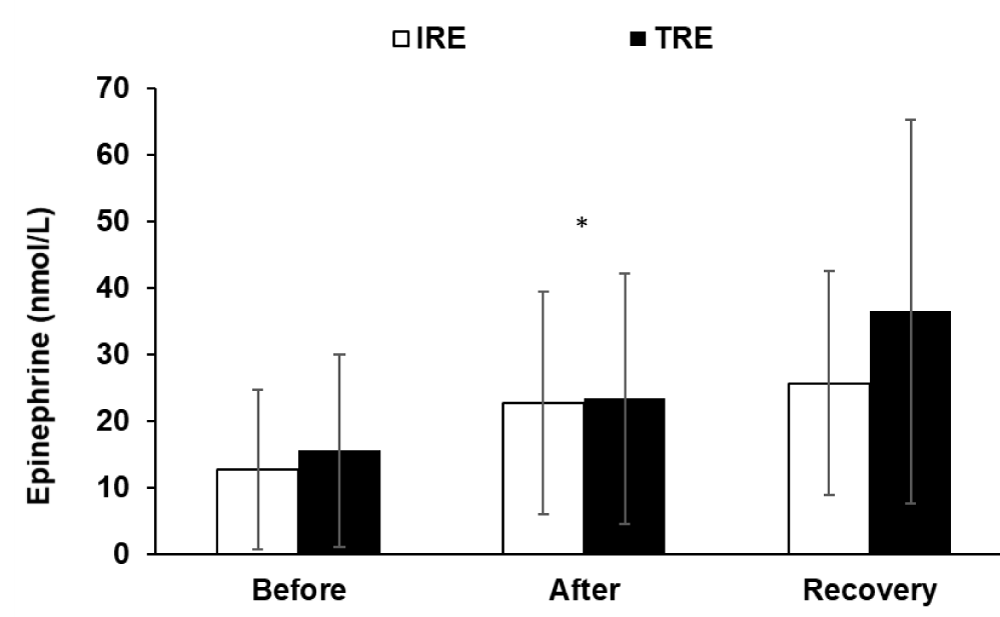
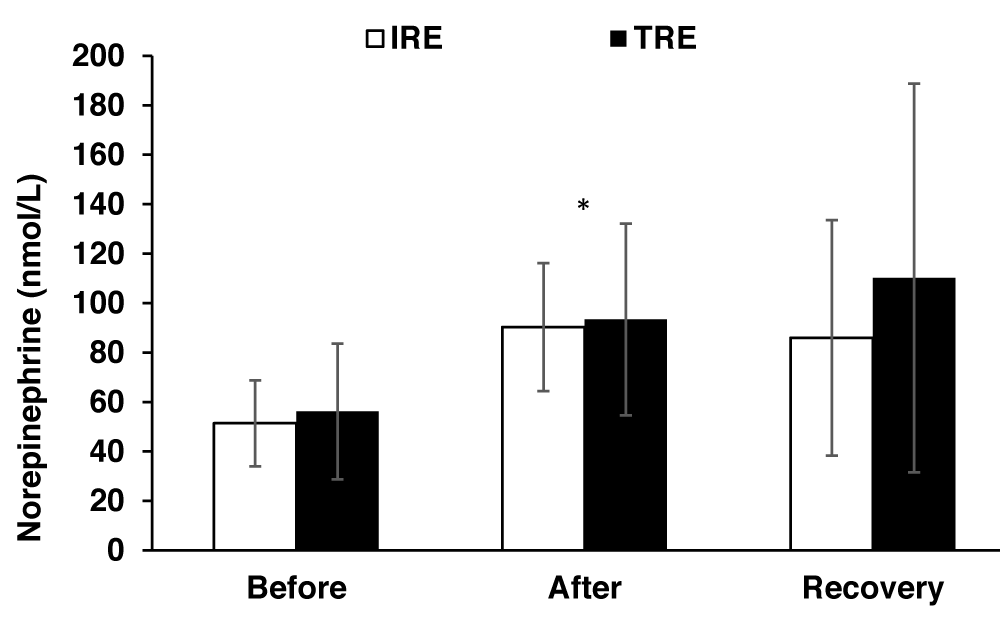
Epinephrine and norepinephrine levels increased significantly (F2, 22 = 7.88, p < 0.01; F2, 22 = 7.3, p < 0.01, respectively) in response to both resistance exercise trials (Figures 3 & 4), though, the differences between the two trials were non-significant.
Figure 3: Epinephrine hormone values (means ± SD) before and after exercise, and after the recovery period for two exercise protocols.* indicates a significant (p < 0.01) exercise effect. IRE, interval resistance exercise. TRE is a traditional resistance exercise.
Figure 4: Norepinephrine hormone values (means±SD) before and after exercise, and after the recovery period for two exercise protocols. * indicates a significant (P<0.01) exercise effect. IRE, interval resistance exercise. TRE is a traditional resistance exercise.
The main findings of the present study were that all variables were increased in response to both resistance exercise trials and that except for growth hormone changes in other variables were not different between the TRE and IRE protocols. These findings are consistent with previous studies that have reported increases in cortisol, GH, and catecholamines after an acute resistance exercise [29,31-34]. For instance, Allman, et al. [31] reported that acute resistance exercise with 65% 1RM with 90 seconds of rest resulted in elevated concentration GH, epinephrine, and norepinephrine concentration. These hormonal changes were associated with increased lipolysis during and after the exercise. The elevation of GH and catecholamines induced by resistance exercise may contribute to the stimulation of lipolysis [31]. Catecholamines regulate the lipolysis pathway by activating adenylyl cyclase through β-adrenoceptors (β-AR), specifically β-AR1-3, while α2-AR subtypes inhibit lipolysis. This signaling pathway increases the concentration of cyclic adenosine monophosphate (cAMP) and activates Protein Kinase A (PKA), which in turn phosphorylates specific serine residues of various proteins to enhance lipolysis [11]. Leite, et al. [34] reported that a single session of resistance exercise with 80% of 1RM and 2 minutes of rest increased cortisol and GH levels. These findings are consistent with those of Kramer, et al. [35], who demonstrated elevated cortisol and GH following four sets of ten repetitions at 1RM with 90 seconds of rest. Cortisol has been shown to stimulate leptin production, which in turn increases the activity of sympathetic nerves and promotes lipolysis [36,37]. Moreover, cortisol may influence the phosphorylation of perilipin, a protein involved in the regulation of lipolysis [38].
In addition to cortisol, GH stimulates lipolysis through several mechanisms: 1) GH increases the sensitivity of adipocyte β-receptor to catecholamines 2) GH stimulates lipolytic enzymes such as hormone-sensitive lipase 3) GH inhibits triglyceride-storing enzymes like lipoprotein lipase, fatty acid synthase, or acetyl-CoA carboxylase [39,40]. Furthermore, Peake, et al. [41] reported increased levels of epinephrine, norepinephrine, cortisol, and GH following a HIIT session. The HIIT session consisted of 10 × 4-minute intervals at 81.6 ± 3.7% of VO2max and 72.0 ± 3.2% of peak power output, with 2 minutes of rest (11.4 ± 0.9% of peak power output) between intervals.
Dote-Montero, et al. [42] emphasized several studies indicating an increase in cortisol levels following an acute HIIT session. The activation of the hypothalamic-pituitary-adrenal axis by stressors like exercise can impact cortisol synthesis [42]. However, our study did not find any significant differences in epinephrine, norepinephrine, and cortisol levels after exercise between the two resistance protocols. These findings align with those of Zarei, et al. [29], who reported non-significant differences in epinephrine response to TRE and HIIRE protocols, possibly attributable to variations in exercise duration or intensity. Nevertheless, our study demonstrated that GH concentration was significantly higher in the HIRE protocol compared to the TRE protocol. Hence, our results are consistent with previous literature indicating that GH response is greater in resistance exercises involving high total work and short rest intervals [24]. Previous research has shown that the most pronounced GH responses occur when resistance exercises are combined with minimal recovery periods. This evidence underscores the undeniable influence of various factors, such as exercise load type, exercise repetitions and rounds, and work-recovery intervals, on GH response [24].
To conclude, the present study found that the recovery type of resistance exercise does not have an impact on lipolytic hormones, except GH. Our results demonstrated that cortisol, epinephrine, and norepinephrine increased following resistance exercise, regardless of the protocols used. These findings suggest that the elevation of lipolytic hormones may contribute to enhanced fat oxidation and lipolysis, given their significant involvement in these processes during and after exercise. Notably, GH exhibited a more pronounced increase in response to active recovery following exercise and may play a substantial role in promoting lipolysis.
The authors would like to express their gratitude to Professor Sajad Ahmadizad for helping out with exercise protocols and to the volunteers for their enthusiastic participation in this study.
Data availability statement
Data generated or analyzed during this study are not publicly available due to confidentiality agreements with research collaborators but are available from the corresponding author upon reasonable request.
- Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord. 2003;27(8):875-888. Available from: https://pubmed.ncbi.nlm.nih.gov/12861227/
- Large V, Peroni O, Letexier D, Ray H, Beylot M. Metabolism of lipids in human white adipocyte. Diabetes Metab. 2004;30(4):294-309. Available from: https://pubmed.ncbi.nlm.nih.gov/15525872/
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383-1386. Available from: https://pubmed.ncbi.nlm.nih.gov/15550674/
- Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. 2014;35(4):473-493. Available from: https://pubmed.ncbi.nlm.nih.gov/24736043/
- Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79-101. Available from: https://pubmed.ncbi.nlm.nih.gov/17313320/
- Kitamura T, Kitamura Y, Kuroda S, Hino Y, Ando M, et al. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol Cell Biol. 1999;19(9):6286-6296. Available from: https://pubmed.ncbi.nlm.nih.gov/10454575/
- Strålfors P, Honnor RC. Insulin-induced dephosphorylation of hormone-sensitive lipase. Correlation with lipolysis and cAMP-dependent protein kinase activity. Eur J Biochem. 1989;182(2):379-385. Available from: https://pubmed.ncbi.nlm.nih.gov/2661229/
- Choi YH, Park S, Hockman S, Zmuda-Trzebiatowska E, Svennelid F, Haluzik M, et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest. 2006;116(12):3240-3251. Available from: https://pubmed.ncbi.nlm.nih.gov/17143332/
- Davy KP, Orr JS. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev. 2009;33(2):116-124. Available from: https://pubmed.ncbi.nlm.nih.gov/18602694/
- Possenti R, Muccioli G, Petrocchi P, Cero C, Cabassi A, Vulchanova L, et al. Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem J. 2012;441(1):511-22. Available from: https://pubmed.ncbi.nlm.nih.gov/21880012/
- Tsiloulis T, Watt MJ. Exercise and the Regulation of Adipose Tissue Metabolism. Prog Mol Biol Transl Sci. 2015;135:175-201. Available from: https://pubmed.ncbi.nlm.nih.gov/26477915/
- Song Y, Altarejos J, Goodarzi MO, Inoue H, Guo X, Berdeaux R, et al. CRTC3 links catecholamine signalling to energy balance. Nature. 2010;468(7326):933-939. Available from: https://pubmed.ncbi.nlm.nih.gov/21164481/
- Morelli M, Gaggini M, Daniele G, Marraccini P, Sicari R, Gastaldelli A. Ectopic fat: the true culprit linking obesity and cardiovascular disease? Thromb Haemost. 2013;110(4):651-660. Available from: https://pubmed.ncbi.nlm.nih.gov/23884194/
- Roh HT, Cho SY, So WY. A Cross-Sectional Study Evaluating the Effects of Resistance Exercise on Inflammation and Neurotrophic Factors in Elderly Women with Obesity. J Clin Med. 2020;9(3):842. Available from: https://pubmed.ncbi.nlm.nih.gov/32244926/
- Melanson EL, Sharp TA, Seagle HM, Donahoo WT, Grunwald GK, Peters JC, et al. Resistance and aerobic exercise have similar effects on 24-h nutrient oxidation. Med Sci Sports Exerc. 2002;34(11):1793-800. Available from: https://pubmed.ncbi.nlm.nih.gov/12439085/
- Melby C, Scholl C, Edwards G, Bullough R. Effect of acute resistance exercise on postexercise energy expenditure and resting metabolic rate. J Appl Physiol (1985). 1993;75(4):1847-1853. Available from: https://pubmed.ncbi.nlm.nih.gov/8282641/
- Chatzinikolaou A, Fatouros I, Petridou A, Jamurtas A, Avloniti A, Douroudos I, et al. Adipose tissue lipolysis is upregulated in lean and obese men during acute resistance exercise. Diabetes Care. 2008;31(7):1397-9. Available from: https://pubmed.ncbi.nlm.nih.gov/18375413/
- Petridou A, Siopi A, Mougios V. Exercise in the management of obesity. Metabolism. 2019;92:163-169. Available from: https://pubmed.ncbi.nlm.nih.gov/30385379/
- Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr Rev. 2018;39(2):79-132. Available from: https://pubmed.ncbi.nlm.nih.gov/29518206/
- Lamotte M, Fleury F, Pirard M, Jamon A, van de Borne P. Acute cardiovascular response to resistance training during cardiac rehabilitation: effect of repetition speed and rest periods. Eur J Cardiovasc Prev Rehabil. 2010;17(3):329-336. Available from: https://pubmed.ncbi.nlm.nih.gov/20104178/
- Fatouros I, Chatzinikolaou A, Paltoglou G, Petridou A, Avloniti A, Jamurtas A, et al. Acute resistance exercise results in catecholaminergic rather than hypothalamic-pituitary-adrenal axis stimulation during exercise in young men. Stress. 2010;13(6):461-8. Available from: https://pubmed.ncbi.nlm.nih.gov/20666650/
- Raymond LM, Renshaw D, Duncan MJ. Acute Hormonal Response to Kettlebell Swing Exercise Differs Depending on Load, Even When Total Work Is Normalized. J Strength Cond Res. 2021;35(4):997-1005. Available from: https://pubmed.ncbi.nlm.nih.gov/30273291/
- Nindl BC, Hymer WC, Deaver DR, Kraemer WJ. Growth hormone pulsatility profile characteristics following acute heavy resistance exercise. J Appl Physiol (1985). 2001;91(1):163-172. Available from: https://pubmed.ncbi.nlm.nih.gov/11408427/
- Bosco C, Colli R, Bonomi R, von Duvillard SP, Viru A. Monitoring strength training: neuromuscular and hormonal profile. Med Sci Sports Exerc. 2000;32(1):202-208. Available from: https://pubmed.ncbi.nlm.nih.gov/10647550/
- Ahmadizad S, Bassami M, Hadian M, Eslami M. Influences of two high intensity interval exercise protocols on the main determinants of blood fluidity in overweight men. Clin Hemorheol Microcirc. 2016;64(4):827-835. Available from: https://pubmed.ncbi.nlm.nih.gov/27802216/
- Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(5):1077-1084. Available from: https://pubmed.ncbi.nlm.nih.gov/22289907/
- Moro T, Tinsley G, Bianco A, Gottardi A, Gottardi GB, Faggian D, et al. High intensity interval resistance training (HIIRT) in older adults: Effects on body composition, strength, anabolic hormones and blood lipids. Exp Gerontol. 2017;98:91-98. Available from: https://pubmed.ncbi.nlm.nih.gov/28821429/
- Paoli A, Moro T, Marcolin G, Neri M, Bianco A, Palma A, et al. High-Intensity Interval Resistance Training (HIRT) influences resting energy expenditure and respiratory ratio in non-dieting individuals. J Transl Med. 2012;10:237. Available from: https://pubmed.ncbi.nlm.nih.gov/23176325/
- Zarei M, Foroozan P, Koushkie Jahromi M, Hemmatinafar M. Acute Effect of High-intensity Interval and Traditional Resistance Training on Lipolysis Factors in Overweight Young Girls. Women’s Health Bulletin. 2021;8(2):83-90. Available from: https://womenshealthbulletin.sums.ac.ir/article_47454.html
- Bassami M, Karimi M, Ahmadizad S. Comparable effects of interval and traditional resistance exercise on lipolysis and insulin concentration in healthy young men. Science & Sports. 2023.
- Allman BR, Morrissey MC, Kim JS, Panton LB, Contreras RJ, Hickner RC, et al. Fat metabolism and acute resistance exercise in trained women. J Appl Physiol (1985). 2019 Mar 1;126(3):739-745. Available from: https://pubmed.ncbi.nlm.nih.gov/30605402/
- Goto K, Ishii N, Kizuka T, Kraemer RR, Honda Y, Takamatsu K. Hormonal and metabolic responses to slow movement resistance exercise with different durations of concentric and eccentric actions. Eur J Appl Physiol. 2009;106(5):731-9. Available from: https://pubmed.ncbi.nlm.nih.gov/19430944/
- Kon M, Ikeda T, Homma T, Akimoto T, Suzuki Y, Kawahara T. Effects of acute hypoxia on metabolic and hormonal responses to resistance exercise. Med Sci Sports Exerc. 2010;42(7):1279-85. Available from: https://pubmed.ncbi.nlm.nih.gov/20019623/
- Leite RD, Prestes J, Rosa C, De Salles BF, Maior A, Miranda H, et al. Acute effect of resistance training volume on hormonal responses in trained men. J Sports Med Phys Fitness. 2011;51(2):322-328. Available from: https://pubmed.ncbi.nlm.nih.gov/21681169/
- Kraemer WJ, Häkkinen K, Newton RU, Nindl BC, Volek JS, McCormick M, et al. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol (1985). 1999;87(3):982-992. Available from: https://pubmed.ncbi.nlm.nih.gov/10484567/
- Bouassida A, Zalleg D, Bouassida S, Zaouali M, Feki Y, Zbidi A, et al. Leptin, its implication in physical exercise and training: a short review. J Sports Sci Med. 2006;5(2):172-81. Available from: https://pubmed.ncbi.nlm.nih.gov/24259989/
- Ramsay TG. Fat cells. Endocrinol Metab Clin North Am. 1996;25(4):847-70. Available from: https://pubmed.ncbi.nlm.nih.gov/8977049/
- Xu C, He J, Jiang H, Zu L, Zhai W, Pu S, et al. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol. 2009;23(8):1161-1170. Available from: https://pubmed.ncbi.nlm.nih.gov/19443609/
- Kraemer WJ, Dunn-Lewis C, Comstock BA, Thomas GA, Clark JE, et al. Growth hormone, exercise, and athletic performance: a continued evolution of complexity. Curr Sports Med Rep. 2010;9(4):242-252. Available from: https://pubmed.ncbi.nlm.nih.gov/20622543/
- Nam SY, Marcus C. Growth hormone and adipocyte function in obesity. Horm Res. 2000;53 Suppl 1:87-97 Available from: https://pubmed.ncbi.nlm.nih.gov/10895049/
- Peake JM, Tan SJ, Markworth JF, Broadbent JA, Skinner TL, Cameron-Smith D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am J Physiol Endocrinol Metab. 2014;307(7):E539-552. Available from: https://pubmed.ncbi.nlm.nih.gov/25096178/
- Dote-Montero M, Carneiro-Barrera A, Martinez-Vizcaino V, Ruiz JR, Amaro-Gahete FJ. Acute effect of HIIT on testosterone and cortisol levels in healthy individuals: A systematic review and meta-analysis. Scand J Med Sci Sports. 2021;31(9):1722-1744. Available from: https://pubmed.ncbi.nlm.nih.gov/34022085/