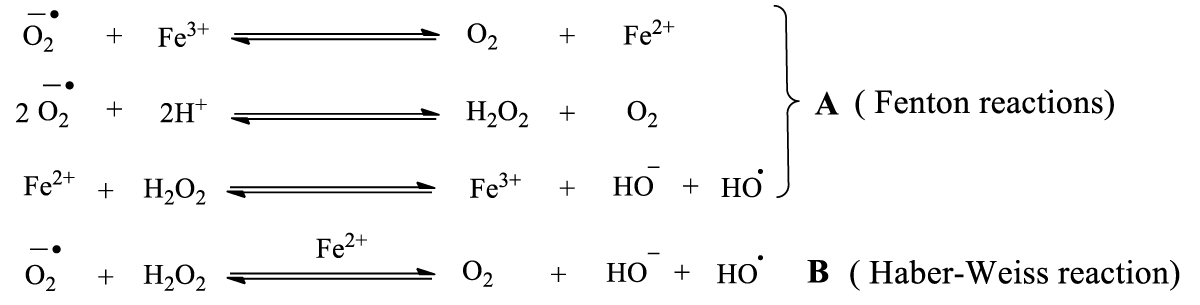More Information
Submitted: April 30, 2024 | Approved: May 14, 2024 | Published: May 15, 2024
How to cite this article: Sow IS, El-Manssouri N, Yang D. Reactive Oxygen Species Production from Hydroxamic Acid and their Iron (III) Complexes against Staphylococcus aureus and Escherichia coli. J Clin Intensive Care Med. 2024; 9: 017-020.
DOI: 10.29328/journal.jcicm.1001048
Copyright license: © 2024 Sow IS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hydroxamic acid; Iron complexes; Reactive Oxygen Species (ROS)
Reactive Oxygen Species Production from Hydroxamic Acid and their Iron (III) Complexes against Staphylococcus aureus and Escherichia coli
Ibrahima Sory Sow1*, Naïma El Manssouri1 and Dong Yang2
1Microbiology, Bioorganic and Macromolecular Chemistry Unit, Faculty of Pharmacy, Université libre de Bruxelles (ULB), 1050 Brussels, Belgium
2Clinical Laboratory, Shanxi Provincial People’s Hospital, Affiliated with Shanxi Medical University, Taiyuan 030001, China
*Address for Correspondence: Ibrahima Sory Sow, Microbiology, Bioorganic and Macromolecular Chemistry Unit, Faculty of Pharmacy, Université libre de Bruxelles (ULB), 1050 Brussels, Belgium, Email: sow79b@gmail.com
The N-hydroxydodecanamide (HA12) and its complexes tri-hydroxamato-iron(III) and di-hydroxamto-iron(III) chloride (HA8Fe3 and HA12Fe3Cl, respectively) showed antibacterial and antimycobacterial activities. The proteomic analysis demonstrated that the targets of Hydroxamic Acid (HA) and their complexes were involved in the biosynthesis of mycobacterial cell walls. The Reactive Oxygen Species (ROS) is one of the key elements to cause oxidative stress, damaging DNA, and cell membranes impaired during the procedure to kill bacteria. Here, the ROS production was determined to evaluate the compounds HA12, HA8Fe3, HA12Fe3Cl, and ZnCl2 against bacteria using 2’,7’-dichlorofluorescein diacetate (DCFDA) by spectrofluorometric analysis. The low fluorescence was observed using the compounds HA12, HA8Fe3, HA12Fe3Cl, and ZnCl2 treating the S. aureus and E. coli, indicating that the ROS production could not be observed using the compounds used at a dose higher than the Minimum Inhibitory Concentration (MIC). It was noted that the ROS determination could be performed with a concentration less than or equal to the MIC. This would enable the mechanism of action linked to the ROS production by HA and their metal complexes to be determined.
Hydroxamic Acid (HA) has many applications in biology and medicine [1], particularly against bacteria, cancer cells, and fungi [2]. Due to the ability to bidentate chelate metal ions, HA displayed multiple biological activities. For instance, they interact with a variety of metal-containing enzymes, such as matrix metalloproteases, lipoxygenase, hydrolase, urease, peptide deformylase, histone deacetylase, carbonic anhydrase to inhibit their activity [3,4].
HA were developed as medicines for the following diseases: cancer, cardiovascular disease, HIV, Alzheimer’s disease, malaria, hypertension, tuberculosis, glaucoma, ulcers, and metal poisoning, including iron. They also were developed as insecticides, antioxidants, anticorrosive agents, and siderophores [4]. The hydroxamate group is considered to be a key element in the pharmacophores [5]. The hydroxyurea was used to treat sickle cell anaemia to induce DNA damage by the production of radicals (nitrogen dioxide radical: •NO2, which is obtained by oxidation of the nitric oxide radical: •NO) [6].
Reactive oxygen species (ROS), an important indicator of ferroptosis, are oxygen and water molecules that have undergone reduction and oxidation, respectively. The redox reaction products include peroxides (•O222ˉ, H2O2), superoxide anion (O2•ˉ), the hydroxyl anion (ˉOH) (Scheme 1), and free radicals (O2•, HO•, RO•, NO•, and NO2•) generated by other various sources [7,8]. The ROS can cause oxidative stress, resulting in DNA, cell membranes, or protein damage. The H2O2 produced by ROS can penetrate the cell membrane and kill bacteria [9].
Scheme 1: Fenton and Haber-Weiss reactions.
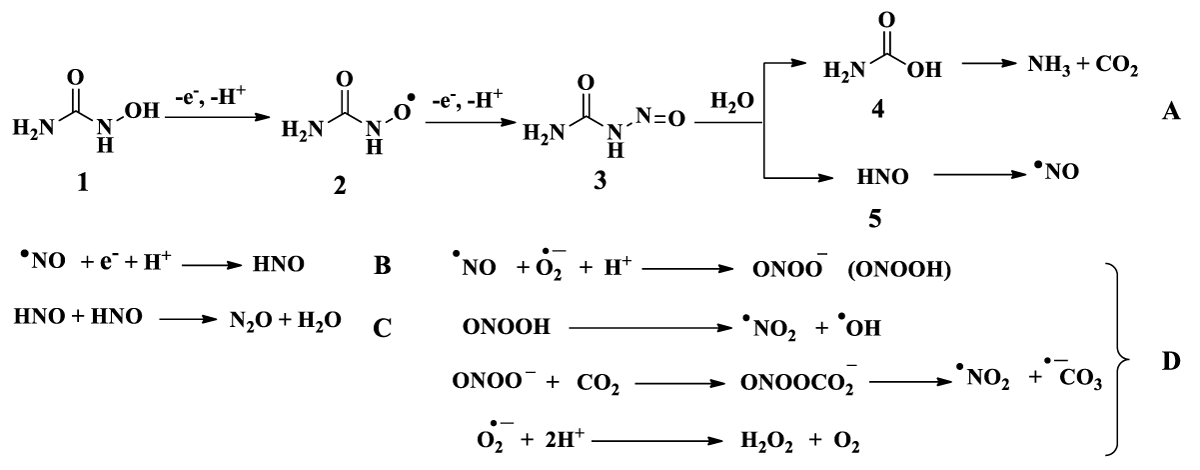
Given the potential value of HA as a therapeutic agent, it would be interesting to understand how NO is released from HA under physiological conditions. It still remains unclear whether it is formed via oxidation of hydroxyurea (1) by heme proteins using hydrogen peroxide (H2O2) (Scheme 2A) [6,10] or by occurrence of other reactions; including the direct release of NO from the nitroxide radical (2) and the dimerisation of nitroxyl HNO (5) to generate nitrous oxide (N2O) (Scheme 2B, 2C) [6,10]. The formation of anionic and radical species (the peroxynitrite anion (ONOOˉ), nitrogen dioxide (•NO2), carbonate anion (CO3•ˉ), and hydrogen peroxide (H2O2) (Scheme 2D) [6].
Scheme 2: Mechanism to release nitric oxide from hydroxyurea (A), N2O formation by HNO dimerisation (B and C), and the formation of anionic and radical species (the peroxynitrite anion (ONOOˉ), nitrogen dioxide (•NO2), carbonate anion (CO3•ˉ) and hydrogen peroxide (H2O2) (D) [6]. 3 = nitrosoformamide, 4 = carbamic acid.
Analytical methods were developed to detect the presence of radicals •NO (spectrophotometry or spectrofluorometry). The •NO led to an increase in fluorescence whereupon the •NO radicals were trapped by the thiol-type antioxidants, and a decrease in fluorescence was observed [11].
Although HA has a broad spectrum of biological activities, its antimicrobial properties are generally enhanced in metallic chelate form [12]. Although the biological properties are attributed to lipophilicity and chelation for HA and the presence of the metal for their complexes, the production of ROS by Fe2O3, Fe3O4, and CuO has been reported [13].
The antimicrobial effects of ZnO nanoparticles are due to the release of Zn2+ ions; leading to ROS production and consequently disruption of bacterial cell walls [13]. The ROS production by Zn2+ ions is not direct, unlike Fe2+ or Cu2+. The first step is an interaction of Zn2+ ions with -SH of the cysteines.
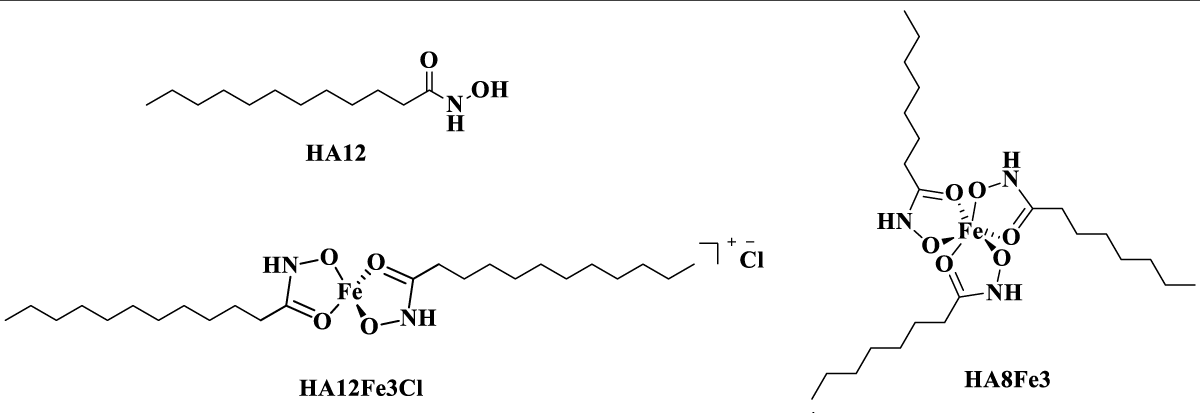
The HA with various carbon chain and their metal ions complexes were synthesised in our previous work [14] and displayed antibacterial and antimycobacterial activity [14,15]. In order to assess the potential interactions between the antibacterial properties and the ROS production for bacteria, we performed the experiment to determine the ROS production using the HA12 and its corresponding Fe(III) complex (HA8Fe3, HA12Fe3Cl) treating bacteria. The chemical structures of HA12 and complexes HA8Fe3 and HA12Fe3Cl are shown in Figure 1.
Figure 1: Chemical structures of N-hydroxydodecanamide (HA12), tri-dodecanohydroxamato-Fe(III) (HA8Fe3) and di-hydroxamato-Fe(III) chloride (HA12Fe3Cl).
Materials (Table 1)
| Table 1: The materials, reagents, and bacterial strains used in the experiments. | ||
| Materials | Catalog numbers | Suppliers |
| Reagents | ||
| Dimethyl sulfoxide (DMSO) | 232861407 | Chem Lab (Zedelgem, Belgium) |
| Zinc chloride (ZnCl2) | 53244 | Merck (Darmstadt, Germany) |
| 2',7'-dichlorofluorescein diacetate (DCFDA) | --- | Sigma Aldrich (Saint Louis, USA) |
| Mueller Hinton Broth (MHB) | 70192 | Sigma Aldrich (Saint Louis, USA) |
| Cetrimide | --- | Cetavlon ICI-Pharma (Destelbergen, Belgium) |
| Material | ||
| 96-well microplates (transparent flat bottom and black side wall) | 7342327 | Sigma Aldrich (Saint Louis, USA). |
| Spectrophotometer | 192736 | Bio-Tek (Winooski, USA) |
| Bacteria strains | ||
| Staphylococcus aureus | LMG 8064 | Microbiology lab at Ghent University, Belgium |
| Escherichia coli | LMG 8223 | Microbiology lab at Ghent University, Belgium |
Methods
The synthetic, characterisation and assessment of biological properties methods were reported in previous work [14]. ROS production was measured using DCFDA. Briefly, an initial solution of 10 millimolar (mM) DCFDA in DMSO was diluted with MHB to obtain a 1 mM solution. In addition, a stock solution of 10 mM in DMSO for the compounds HA12, HA8Fe3, HA12Fe3Cl, or ZnCl2 was prepared. Then, 10 µL DCFDA at 1 mM and 25 µL compound with 10 mM were added to obtain a final volume of 1 mL bacterial culture. The final concentration of DCFDA and compound in bacteria culture are 10 µM and 250 µM respectively.
For the positive control, 10 µL of DCFDA at 1 mM and 10 µL of cetrimide at 0.59 mM (0.2 mg/mL) were added to complete 1 mL of bacterial culture. The final concentration of DCFDA and cetrimide in bacteria culture are 10 µM and 5.9 µM (2 µg/mL) respectively. The loaded culture was incubated at 37 °C for 1 h before measuring fluorescence (excitation and emission at 485 nm and 530 nm, respectively) using a Biotek plate reader spectrophotometer. The measurement was carried out in 96-well plates (transparent flat bottom and black side wall) [16].
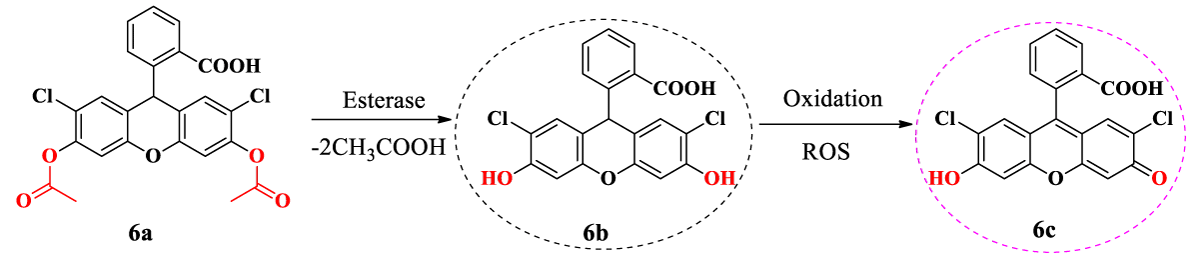
The DCFDA (6a) is a non-fluorescent dye, which is hydrolysed intracellularly by esterases into its polar but non-fluorescent form (6b). The compound 6b was oxidated to produce ROS and other intracellular peroxides transformations were obtained in the highly fluorescent form (6c, Scheme 3)[17]. Here, we tried to determine the ROS production in bacteria (S. aureus and E. coli) treated by compound 6a. The control groups in the presence and absence of compounds (negative control) were also performed. Fluorescence was recorded on a fluorescence spectrophotometer and expressed as a percentage of fluorescence to the negative control.
Scheme 3: Mechanism of action of DCFDA in bacteria cell [17]. All experiments were carried out at the Microbiology, Bioorganic, and Macromolecular Chemistry Laboratory, Faculty of Pharmacy, Université libre de Bruxelles in 2022.
All experiments were carried out at the Microbiology, Bioorganic, and Macromolecular Chemistry Laboratory, Faculty of Pharmacy, Université libre de Bruxelles in 2022.
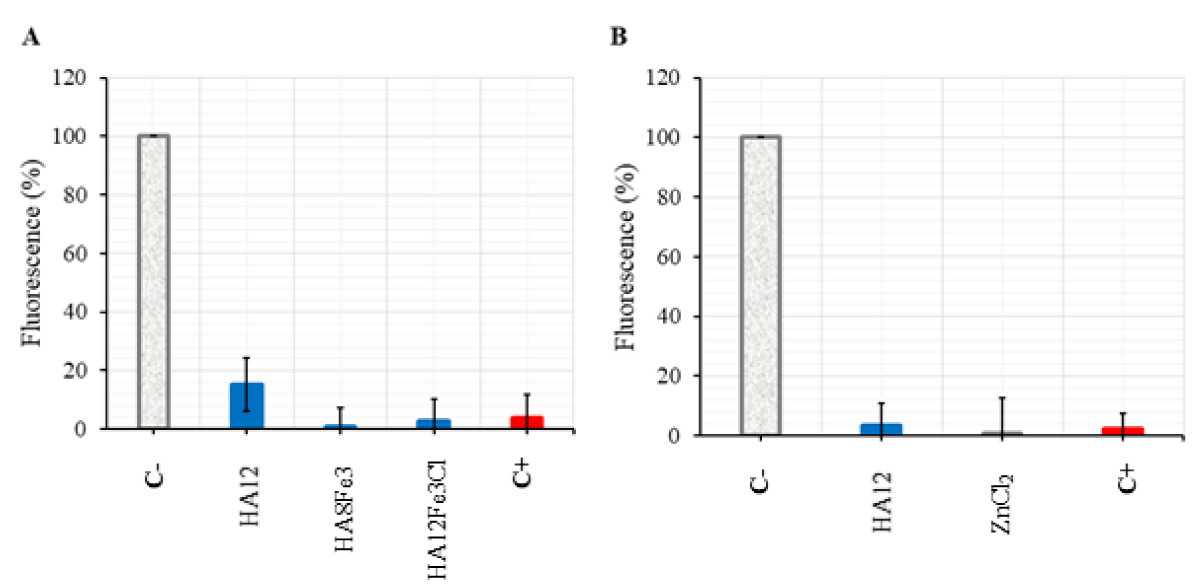
In the present study, the compounds HA12, HA8Fe3, HA12Fe3Cl with 250 µM and cetrimide with 5.9 µM (2 µg/mL) treating S. aureus gave low percentage values of fluorescence: 15.16%, 0.77%, 2.88% and 4.03%, respectively (Figure 2A).A similar sharp decrease in percentage fluorescence values was also observed for the compounds HA12 and ZnCl2 at 250 µM against E. coli (3.26 and 0.69%, respectively) (Figure 2B).
Figure 2: Determination of ROS production in S. aureus with the compounds HA12, HA8Fe3, HA12Fe3Cl (A) and E. coli with the compounds HA12, ZnCl2 (B). C- = Negative control (S. aureus or E. coli + DCFDA), C+ = Positive control (cetrimide at the concentration of 2 µg/mL). The experiment was carried out three times, each in triplicate.
It was reported that the decrease in fluorescence tested by spectrophotometry probably reflects the non-conversion of •NO into ONOOˉ for compound HA12 [11]. The action mechanism of compounds Fe2O3, Fe3O4, and ZnO against bacteria is due to the ROS production, the compounds HA8Fe3, HA12Fe3Cl, and ZnCl2 did not produce ROS in the experimental condition in the present study.
In previously published work, compound HA12 showed an inhibitory effect against S. aureus and E. coli strains with a MIC equal to 62.5 µM [14]. As for the compound HA8Fe3, MIC of 78.13 µM and 156.25 µM against S. aureus and E. coli respectively were observed. The bactericidal effects of compounds HA12 and HA8Fe3 were also observed with minimum bactericidal concentration (MBC) values equal to 125 µM against S. aureus and E. coli [14]. MBC were obtained with the compound HA8Fe3 against S. aureus and E. coli with values of 156 µM and 312 µM respectively. The MIC obtained with ZnCl2 against E. coli was 78.13 µM and the MBC was 312 µM [14]. These previously worked MBC results support our hypothesis that ROS production could not be observed at a concentration that would immediately kill the bacteria.
We believe that the compounds tested were applied at a concentration that appears to have killed the bacteria. It is probably due to the fact the bacteria were killed at a higher concentration of compounds. When the bacteria died, ROS were no longer produced. Therefore, it should be noted that future experiments should be performed at lower concentrations less than or equal to the MIC to determine the ROS production.
The low fluorescence values compared with the control in the spectrofluorometric analysis indicate a lack of ROS production. The ROS production in S. aureus and E. coli was not observed in the experimental conditions using the compounds HA12, HA8Fe3, HA12Fe3Cl, and ZnCl2 at the concentration of 250 µM. The lack of ROS production was probably due to the high concentration used in the experiment which resulted in killing the bacteria directly. If bacteria die, the compounds can no longer produce ROS. Spectrofluorometric analysis to determine ROS production should be carried out at a concentration range below 250 µM.
Author contributions
Conceptualization, writing-original draft I.S.S., methodology, analysis I.S.S. and N.E.M; review, D.Y.
All authors have read and agreed to the published version of the manuscript.
I am grateful to Prof. F. Dufrasne, Prof. M. Gelbecke, and Prof. V. Fontaine for their help and fruitful discussions. I thank the ULB cooperation service for granting me a scholarship to complete my doctoral thesis and Abdoulaye Yéro Baldé, the former minister of higher education and scientific research of Guinea for his support allowing me to begin my doctoral research.
- Khan S, P MR, Rizvi A, Alam MM, Rizvi M, Naseem I. ROS mediated antibacterial activity of photoilluminated riboflavin: A photodynamic mechanism against nosocomial infections. Toxicol Rep. 2019 Jan 9; 6:136-142. doi: 10.1016/j.toxrep.2019.01.003. PMID: 30671349; PMCID: PMC6330557.
- Kumar H, Gupta S, Siddiqui A, Kumar V. Advances in Design and Development of Inhibitors of Nitric Oxide Synthases, Curr. Enzym. Inhib. 2013; 9:117–141. https://doi.org/10.2174/1573408011309020005.
- Saban N, Bujak M. Hydroxyurea and hydroxamic acid derivatives as antitumor drugs. Cancer Chemother Pharmacol. 2009 Jul;64(2):213-21. doi: 10.1007/s00280-009-0991-z. Epub 2009 Apr 7. PMID: 19350240.
- Gupta SP, Sharma A. Hydroxamic acids: A unique family of chemicals with multiple biological activities, Springer-Verlag. 2013; 1–17.
- Bertrand S, Hélesbeux JJ, Larcher G, Duval O. Hydroxamate, a key pharmacophore exhibiting a wide range of biological activities. Mini Rev Med Chem. 2013 Jul;13(9):1311-26. doi: 10.2174/13895575113139990007. PMID: 23701657.
- Burkitt MJ, Raafat A. Nitric oxide generation from hydroxyurea: significance and implications for leukemogenesis in the management of myeloproliferative disorders. Blood. 2006 Mar 15;107(6):2219-22. doi: 10.1182/blood-2005-08-3429. Epub 2005 Nov 10. PMID: 16282342.
- Kim KM, Cho SS, Ki SH. Emerging roles of ferroptosis in liver pathophysiology. Arch Pharm Res. 2020 Oct;43(10):985-996. doi: 10.1007/s12272-020-01273-8. Epub 2020 Oct 20. PMID: 33079307.
- Lin LS, Song J, Song L, Ke K, Liu Y, Zhou Z, Shen Z, Li J, Yang Z, Tang W, Niu G, Yang HH, Chen X. Simultaneous Fenton-like Ion Delivery and Glutathione Depletion by MnO2 -Based Nanoagent to Enhance Chemodynamic Therapy. Angew Chem Int Ed Engl. 2018 Apr 23;57(18):4902-4906. doi: 10.1002/anie.201712027. Epub 2018 Mar 23. PMID: 29488312.
- Dimapilis EAS, Hsu CS, Mendoza RMO, Lu MC. Zinc oxide nanoparticles for water disinfection, Sustain. Environ. Res. 2018; 28: 47–56. https://doi.org/10.1016/j.serj.2017.10.001.
- Huang J, Sommers EM, Kim-Shapiro DB, King SB. Horseradish peroxidase catalyzed nitric oxide formation from hydroxyurea. J Am Chem Soc. 2002 Apr 3;124(13):3473-80. doi: 10.1021/ja012271v. PMID: 11916434.
- Can Z, Keskin B, Üzer A, Apak R. Detection of nitric oxide radical and determination of its scavenging activity by antioxidants using spectrophotometric and spectrofluorometric methods. Talanta. 2022 Feb 1;238(Pt 1):122993. doi: 10.1016/j.talanta.2021.122993. Epub 2021 Oct 22. PMID: 34857326.
- Hassan AU, Sumrra SH, Imran M, Chohan ZH. New 3d multifunctional metal chelates of sulfonamide: Spectral, vibrational, molecular modeling, DFT, medicinal and in silico studies, J. Mol. Struct. 2022; 1254:132305. https://doi.org/10.1016/j.molstruc.2021.132305.
- Aderibigbe BA. Metal-Based Nanoparticles for the Treatment of Infectious Diseases. Molecules. 2017 Aug 18;22(8):1370. doi: 10.3390/molecules22081370. PMID: 28820471; PMCID: PMC6152252.
- Sow IS, Gelbcke M, Meyer F, Vandeput M, Marloye M, Basov S, Van Bael MJ, Berger G, Robeyns K, Hermans S, Yang D, Fontaine V, Dufrasne F. Synthesis and biological activity of iron(II), iron(III), nickel(II), copper(II) and zinc(II) complexes of aliphatic hydroxamic acids, J. Coord. Chem. 2023; 76:76–105. https://doi.org/10.1080/00958972.2023.2166407.
- Yang D, Zhang Y, Sow IS, Liang H, El Manssouri N, Gelbcke M, Dong L, Chen G, Dufrasne F, Fontaine V, Li R. Antimycobacterial Activities of Hydroxamic Acids and Their Iron(II/III), Nickel(II), Copper(II) and Zinc(II) Complexes. Microorganisms. 2023 Oct 23;11(10):2611. doi: 10.3390/microorganisms11102611. PMID: 37894269; PMCID: PMC10609363.
- Zhang XF, Shen W, Gurunathan S. Biologically Synthesized Gold Nanoparticles Ameliorate Cold and Heat Stress-Induced Oxidative Stress in Escherichia coli. Molecules. 2016 Jun 4;21(6):731. doi: 10.3390/molecules21060731. PMID: 27271586; PMCID: PMC6273942.
- Rajneesh, Pathak J, Chatterjee A, Singh SP, Sinha RP. Detection of Reactive Oxygen Species (ROS) in Cyanobacteria Using the Oxidant-sensing Probe 2',7'-Dichlorodihydrofluorescein Diacetate (DCFH-DA). Bio Protoc. 2017 Sep 5;7(17):e2545. doi: 10.21769/BioProtoc.2545. PMID: 34541194; PMCID: PMC8413551.