More Information
Submitted: April 20, 2023 | Approved: May 05, 2023 | Published: May 08, 2023
How to cite this article: German CM, Matias T, Pedro R, Laura S, Martin OS, et al. Peripheral perfusion index in critically ill COVID-19 and its association with multiorgan dysfunction. J Clin Intensive Care Med. 2023; 8: 004-013.
DOI: 10.29328/journal.jcicm.1001043
Copyright License: © 2023 German CM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: COVID-19; Multiorgan dysfunction; Peripheral perfusion index; Outcomes; Mortality; Critically ill
Peripheral perfusion index in critically ill COVID-19 and its association with multiorgan dysfunction
Cornu Matias German1*, Tonelier Matias1, Roel Pedro1, Sanhueza Laura1, Orozco Sergio Martin1, Sepulveda Mariana Elizabet1, Svampa Silvana Enrica1, Arana Osorio Erick1 and Martinuzzi Andres Luciano Nicolas1
1Intensive Care Unit, CMIC, Neuquén, Argentina
2Argentinian Society of Intensive Care, Former Chairman of COSONUME-SATI, Argentina
*Address for Correspondence: Cornu Matias German, Intensive Care Unit, CMIC, Neuquén, Argentina, Email: matiascornu1968@gmail.com
Introduction: Severe cases of COVID-19 presented a high incidence of multi-organ dysfunction syndrome (MODS) during their evolution. This was attributed to a theoretical cytokine storm, where microcirculatory disorders would play a fundamental role, causing these patients to present a sepsis-like pattern as observed in sublingual microcirculation studies. The evidence in this regard is controversial. The Peripheral Perfusion Index is a reliable method to continuously and non-invasively assess the microcirculatory bed, which assesses the pulsatile (PPI) component of the plethysmographic pulse curve.
Methods: We conducted a prospective observational study to evaluate the behavior of the PPI in patients with severe respiratory failure due to SARS-CoV-2 and its association with SDOM.
Results: We evaluated 60 patients with APACHE II 14.6 ± 4.4 and SOFA 4.7 ± 2.1. 55% of the patients presented SDOM. Perfusion monitoring showed IP values of 5.32 ± 1.87 that were associated with normal lactate levels of 1.49 mmol/L (min 0.89/ max 2.20 mmol/L). The PPI values between the living and the dead did not show a significant difference (p = 0.854) or the presence of SDOM.
The PPI values between the patients who presented renal failure, hemodynamics, or perfusion disorders were determined by the presence of hyperlactatemia, and for those who did not present these characteristics, no statistical difference was found; neither when stratified by PaFiO2 ratio. Mortality was 55%.
Conclusion: In our series of patients with severe pneumonia due to COVID-19, we found high PPI values, which would correspond to a pattern of capillary recruitment, and the associated organ injury could not be substantiated by this phenomenon.
The data reported during the SARS-CoV-2 pandemic showed a mortality rate between 30% - 50% in those patients who presented their most severe forms requiring intensive care [1]. This high mortality rate was associated in most publications with the development of multi-organ dysfunction during the disease [2,3].
Although to date, the mechanisms involved in the pathophysiology of Multiorgan Dysfunction Syndrome associated with COVID-19 have not been clearly defined. There are controversies about whether the target organ damage is the consequence of an excessive inflammatory response to the infection called Cytokine Storm or whether it would correspond to direct viral injury in organs with a high density of Angiotensin-converting enzyme 2 (ACE2) receptors or a combination of both [4,5].
Multiorgan dysfunction syndrome has been widely studied concerning septic states where microcirculation plays a central role in its pathophysiology, where inflammatory mediators would be responsible for these microcirculatory alterations [6,7].
In recent years, microcirculation monitoring has been the subject of study concerning the evaluation of tissue perfusion in shock states through different techniques and technologies. Among these monitoring methods, sublingual microcirculation has been used in multiple investigations to assess behavior in patients with critically ill COVID-19 [8-11]. Many of these studies conclude that microcirculation disturbances observed would represent the engine of the cascade of events that lead to multi-organ dysfunction. Among these findings, alterations such as increased capillary density are described, which are not compatible with other states of shock, particularly in septic patients.
In the same way, a series of pathological anatomies from autopsies of COVID-19 patients showed neoformation vessels due to intussusceptive angiogenesis 2.7 times greater than in H1N1 patients [12,13]. These vascular lesions are found in the lung, heart, liver, kidney, brain, and lymphoreticular organs [14], Organs with a high density of ACE2 receptors. These anatomopathological findings and the evidence of increased capillary density obtained by microcirculatory monitoring could correspond to receptor-mediated viral injury and be one of the mechanisms responsible for the microcirculatory pattern and organ injury.
Peripheral Perfusion Index (PPI) obtained by pulse plethysmography has been described as a non-invasive, continuous, bedside option for assessing peripheral microcirculation. This monitoring method was validated in multiple works as a surrogate for microcirculation and tissue perfusion [15-17] and could be useful to assess microcirculatory behavior in COVID-19 patients with organ dysfunction.
We have designed an observational study to evaluate the behavior of the peripheral perfusion index in critically ill COVID-19 and its association with multi-organ dysfunction syndrome and overall mortality.
We conducted a prospective, observational study. The objective of evaluating the behavior of the PPI in patients with severe respiratory failure due to SARS-CoV-2. Patients admitted to the ICU enrolled between April and August 2021.
Inclusion criteria
1) Over 18 years of age, 2) Diagnosis of ARDS secondary to severe pneumonia due to COVID-19 that required mechanical ventilatory assistance (MVA), 3) With a positive result by polymerase chain reaction (PCR) by nasopharyngeal swab. 4) Patients who reached hemodynamic stability after adequate crystalloid and vasoactive resuscitation.
All enrolled patients were followed up for 28 consecutive days to record the data necessary for the analysis of the primary outcomes.
Primary outcomes: Evaluate the behavior of the PPI of patients with severe respiratory failure due to SARS-CoV-2.
Secondary Outcomes: Evaluate the association of PPI values in critically ill COVID-19 with multi-organ dysfunction syndrome.
Data collection: 24 hours after admission and having achieved hemodynamic stability, the following data were recorded for each patient:
General data: gender, age, ICU admission date, and ICU discharge date.
Admission severity scores: APACHE II and SOFA.
Inflammatory parameters at admission: Ferritin, D-dimer, CRP, LDH.
Hemodynamic parameters: Heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP).
Respiratory parameters: Ratio arterial pressure of O2/Fraction of inspired O2.
Renal function parameters: Urea, Creatinine, Urinary volume.
Perfusion Parameters: Lactate, Peripheral Perfusion Index (PPI).
Results: Discharge, death, 28-day mortality, and length of stay (LOS) in the ICU.
Definitions
Hemodynamic stability: State in which the macro-hemodynamic values of blood pressure and heart rate are within normal ranges.
Shock: State of hypoperfusion associated with hyperlactatemia.
Multiorgan Dysfunction (MOD): Understood as dysfunction of 2 or more organs that require external intervention to maintain homeostasis, assessed using the SOFA score.
Acute kidney injury (AKI): Classified according to KDIGO AKI Score stages.
Liver dysfunction: Defined by elevated Bilirubin values according to SOFA (Total Bilirubin > 1,2 mg/dL).
Technique for peripheral perfusion measurement: This was evaluated 24 hours after the start of mechanical ventilation and during the period of hemodynamic stability using PI measured by the plethysmographic curve of the pulse oximetry sensor, from Beneview Monitors, N series; Mindray Bio-Medical Electronics Co.
Treatment standards applied to study patients with severe respiratory failure due to SARS-CoV-2: All patients were intubated and connected to mechanical ventilation. If they had PaFiO2 < 120, they were ventilated in the prone position, received sedation and analgesia, and neuromuscular blockers were used if necessary. All received therapy with low molecular weight heparin and dexamethasone [18].
Statistical analysis
The collected data were stored in an electronic database, and then there were statistically analyzed with the IBM© SSPS 24.0 tool. Baseline characteristics and outcomes were analyzed depending on the nature of the variables. The normality of quantitative data was assessed by Mean, Median, Mode, and Kurtosis with a standard deviation (SD) or a confidence interval of 95% (CI 95%). Also, the Shapiro test for normality was performed. Quantitative data were described with mean and 95% confidence interval. Normally distributed data were analyzed with a 2-tailed t - test (p = 0.05). Otherwise, the Wilcoxon rank sum test was used (p = 0.05). For categorical variables, the Chi-Square test was used. Kruskal Wallis test was used if the variable had 3 or more options.
The sample was segmented to analyze the relationship between PPI and different situations. The segmentation was performed according to the following criteria: Alive-dead within 28 days; Presence or absence of hyperlactatemia; renal failure; Use of Noradrenaline; and finally and stratification was performed in ranges of < 100, 100-200, 200-300, and > 300 according to PaFIO2 to evaluate the correlation of the PPI with those.
During the study period, 66 patients were eligible to be included, 63 met the inclusion criteria, and 3 did not. During the study, 3 more patients had to be excluded due to not reaching the minimal data required to analyze primary outcomes. Therefore, only 60 patients completed the study (Figure 1).
Figure 1: Patient flow chart.
The average age was 54.9 ± 10.9 years, the Apache II at admission was 14.6 ± 4.4 and the SOFA average was 4.7 ± 2.1.
Table 1 shows the clinical and laboratory characteristics of the patients admitted for COVID-19. Among the associated comorbidities we found 65% obesity (n = 39), 43.3% arterial hypertension (n = 26), 25% diabetes (n = 15), and 16.7% COPD (n = 10) as the most frequent.
| Table 1: Clinical and laboratory characteristics of the patients. | |
| N = 60 | |
| Age (years) | 54.97 ± 10.9 |
| Gender Female(F): Male(M) | F 33,3% (20) : M 66,7% (40) |
| Apache II | 14.67 ± 4.4 |
| CRP C-Reactive Protein (mg/l) | 95.9 ± 45.5 |
| SOFA | 4.73 ± 2.13 |
| Fibrinogen (mg/dl) | 748.2 ± 270.4 |
| D-dimer (ng/ml) | 2012 ± 2866.1 |
| Ferritin (ng/ml) | 742.4 ± 270.2 |
| Lactate dehydrogenase LDH (U/l) | 969.4 ± 463.9 |
| Lactic acid plasma level (mmol/l) | 1.49 ± 031 |
| Comorbidities HBP DBT OBESITY COPD |
43.3% (26) 25% (15) 65% (39) 16.7% (10) |
| Multiple organ dysfunction | 55% |
| Renal failure without AKI AKI I AKI II AKI III HEMODIALYSIS |
42.4% (25) 28.8% (17) 6.8% (4) 6.8% (4) 16.9% (10) |
| Hematologic Failure | 28.3% (17) |
| Liver failure | 20% (12) |
| Hemodynamic instability | 41.7% (25) |
| HEART RATE HR | 83.9 ± 20.3 |
| Systolic Blood Pressure SBP mmHg | 122.2 ± 10.3 |
| Diastolic Blood Pressure PAD mmHg | 70.3 ± 11.4 |
| Mean Blood Pressure MAP mmHg | 85.3 ± 8.5 |
| Dose Noradrenaline ND G/Kg/min | 0.11 ± 0.17 (n = 25) |
| Respiratory failure | 100% (60) |
| Partial Pressure of Oxygen PO2 mmHg | 96.5 ± 14.1 |
| Partial Pressure of Carbon Dioxide PCO2 mmHG | 43.6 ± 7.61 |
| PAFIO2 | 169 ± 37.7 |
| Perfusion Index | 5.32 ± 1.87 |
| MV DAYS | 16.9 ± 12.4 |
| FREE DAYS OF MV | 3.4 (0-34) |
| LOS ICU (Days) | 20.3 ± 15.7 |
| Results 28 days Alive Dead |
45% (27) 55% (33) |
| PAFIO2: Arterial Oxygen Pressure/Fraction Of Inspired Oxygen. MV: Mechanical Respiratory Assistance; ICU: Intensive Care Unit; AKI: Acute Kidney Injury; HBP: High Blood Pressure; DBT: Diabetes, COPD: Chronic Obstructive Pulmonary Disease. | |
55% (n = 33) presented multi-organ dysfunction with an average SOFA at the admission of 5.4 ± 2.47. The dysfunction most frequently associated with ARDS secondary to COVID-19 was renal 60% (n = 36); distributed according to KDIGO severity as AKI I 28.3%, AKI II 8.3%, AKI III 6.7%; with a 16.7% requirement for hemodialysis. Hemodynamic failure was the second in frequency of presentation at 41.7% (n = 25) with low Noradrenaline requirements (0.11 ug/kg/min ± 0.17). To a lesser extent, it was associated with other failures (hematological 28.3% n = 17; hepatic 20% n = 12).
Perfusion monitoring showed IP values of 5.32 ± 1.87 in COVID-19 patients. These perfusion data were associated with normal serum lactate levels of 1.49 mmol/L (min 0.89/ max 2.20 mmol/L).
When segmenting the study population into Alive (n = 27) and dead (n = 33), the PPI values were 5.37 ± 1.93 versus 5.28 ± 1.84 p = 0.854. Non-significant differences were found regarding the presence of comorbidities. Regarding the incidence of DOM, there were no significant differences between the living and the dead. The presence of acute renal failure was more frequent in deceased COVID-19 patients than in living ones. As other data to be highlighted, the deceased in the sample presented a higher LDH value, a higher dose of norepinephrine, and a higher partial pressure.
Overall mortality at 28 days was 55%. The deceased in the study had a higher LDH value, a higher dose of norepinephrine, a higher partial pressure of CO2 (PCO2), fewer ARM-free days, and shorter hospitalizations (See Table 2 Alive-Dead).
| Table 2: Segmenting the study population into alive and dead. | |||
| Dead/Alive | Dead N = 33 | Alive N = 27 | p |
| Age (years) | 57.1 ± 9,9 | 52.3 ± 11.6 | |
| Gender Female(F): MALE(M) | F 33.3% (11) : M 66.7% (22) | F 33.3% (9) : M 66.7% (18) | 1 |
| Apache II | 14.9 ± 4,5 | 14.3 ± 4.3 | 0.602 |
| CRP C-Reactive Protein (mg/l) | 97.3 ± 44.5 | 94.4 ± 47.5 | 0.808 |
| SOFA | 5.1 ± 2.4 | 4.3 ± 1.6 | 0.147 |
| Fibrinogen (mg/dl) | 736.6 ± 284.1 | 760.8 ± 260.4 | 0.734 |
| D-dimer (ng/ml) | 2061.9 ± 2763.5 | 1968.5 ± 3009.5 | 0.901 |
| Ferritin (ng/ml) | 749.7 ± 303.5 | 733.4 ± 276.9 | 0.831 |
| Lactate dehydrogenase LDH (U/l) | 1090 ± 501.4 | 843.5 ± 393.4 | 0.041 |
| Lactic acid plasma level (mmol/l) | 1.52 ± 0.3 | 1.45 ± 0.33 | 0.393 |
| Comorbidities HBP DBT OBESITY COPD |
51.5% (17) 21.2% (7) 72.7% (24) 15.2% (5) |
33.3% (9) 29.6% (8) 55.6% (15) 18.5% (5) |
0.164 0.458 0.171 0.735 |
| Multiple organ dysfunction | 60.6% (20) | 48.1% (13) | 0.337 |
| Renal failure without AKI AKI I AKI II AKI III HEMODIALYSIS |
30.3% (10) 30.3% (10) 9.1% (3) 12.1% (4) 18.2 (6) |
55.6% (15) 25.9% (7) 3.7% (1) - 14.8% (4) |
0.049 0.709 0.408 0.063 0.727 |
| Hematologic Failure | 24.2% (8) | 33.3% (9) | 0.441 |
| Liver failure | 21.2% (7) | 18.5% (5) | 0.796 |
| Hemodynamic instability | 51.5% (17) | 29.6% (8) | 0.089 |
| HEART RATE HR | 86.1 ± 17 | 81.2 ± 23.7 | 0.337 |
| Systolic Blood Pressure SBP mmHg | 121.9 ± 9.5 | 122.5 ± 11.3 | 0.711 |
| Diastolic Blood Pressure PAD mmHg | 68.6 ± 13.6 | 72.5 ± 7.5 | 0.188 |
| Mean Blood Pressure MAP mmHg | 84.1 ± 8.3 | 86.8 ± 8.7 | 0.224 |
| Dose Noradrenaline ND G/Kg/min | 0.13 ± 0.2 | 0.05 ± 0.03 (n=8) | 0.044 |
| Respiratory failure | 100% (33) | 100% (27) | 1 |
| Partial Pressure of Oxygen PO2 mmHg | 93.6 ± 14.5 | 100.1 ± 13.3 | 0.078 |
| Partial Pressure of Carbon Dioxide PCO2 mmHG | 46.2 ± 8.1 | 40.5 ± 5.6 | 0.003 |
| PAFIO2 | 154.9 ± 38.7 | 186.3 ± 29.5 | 0.001 |
| Perfusion Index | 5.28 ± 1.84 | 5.37 ± 1.93 | 0.854 |
| MV DAYS | 13.6 ± 5.7 | 20.9 ± 16.7 | 0.022 |
| Free Days OF MV | 1.03 (0-20) | 6.3 (0-34) | 0.0006 |
| LOS ICU (Days) | 14.6 ± 7.6 | 27.1 ± 20.1 | 0.001 |
When the PPI values were analyzed between the patients who presented renal failure, hemodynamics, or perfusion disorders determined by the presence of hyperlactatemia and those who did not present these phenomena, no statistical difference was found (Table 3 PPI values according to characteristics).
| Table 3: Peripheral perfusion index values and organ dysfunction. | |||
| Alive (n = 27) | Dead (n = 33) | p | |
| PPI | 5.37 ± 1.93 | 5.28 ± 1.84 | 0.854 |
| AKI NO (n 24) | AKI YES (n 36) | P | |
| PPI | 5.18 ± 2.06 | 5.41 ± 1.76 | 0.645 |
| VASOACTIVE DRUGS NO (n 36) | VASOACTIVE DRUGS YES (n 24) | P | |
| PPI | 5.29 ± 1.84 | 5.37 ± 1.94 | 0.872 |
| LACTATE <1.8 (n 49) | LACTATE >1.8 (11) | P | |
| PPI | 5.31 ± 1.87 | 5.35 ± 2.23 | 0.951 |
| PPI: Peripheral Perfusion Index; AKI: Acute Kidney Injury | |||
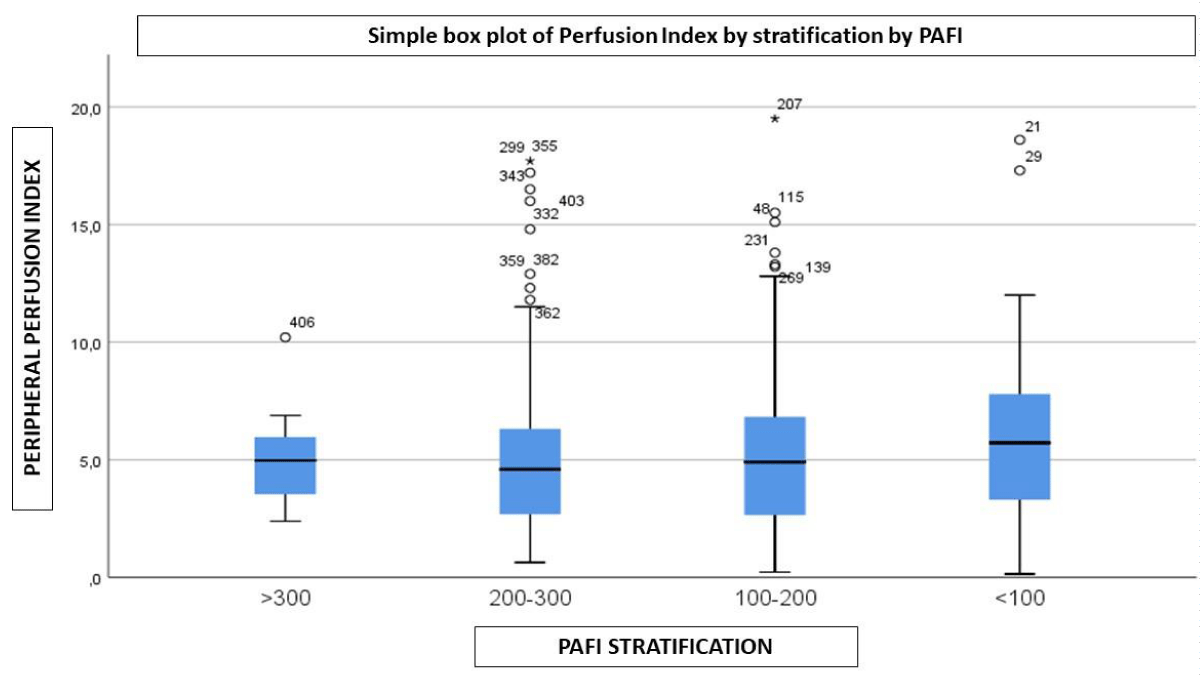
The samples were stratified into 4 groups according to the oxygenation parameters of the SOFA Score. No significant PPI variation was observed between the groups (Figure 2).
Figure 2: PaFio2 – PPI.
Our study evaluated peripheral perfusion using IPP by pulse oximetry in patients with ARDS secondary to severe pneumonia due to COVID-19 to determine its probable relationship with the development of MFO associated with this pathology. The PPI values found in our study were higher than those established as a cut-off point of 1.4 as the normality value of the study by Bakker, et al in patients with septic shock [15] and even higher for established values of 0.6 as a marker of hypoperfusion [19]. Recently, a study reported high PPI values in COVID-19 patients assisted in the emergency department. They established a cut-off point to predict disease severity when the PPI was over 2.2 (non-severe PPI 1.44 ± 1.12; severe PPI 3.69 ± 2.51). These values are lower than in our study (PPI 5.32 ± 1.87). These could be explained by the fact that they used a single measure at admission as isolated data; in our study, we took several measurements during respiratory failure [20].
Consistent with the observed pattern, we found low lactate values, with normal blood pressure values with low vasoactive requirements and low doses, showing a clear difference from what was found in septic shock. These parameters did not present significant differences when compared between the living and the dead. The findings are similar to the values published in other series (Table 4).
In the same way, a recent work [23] evaluated microcirculation, endothelium, and inflammation in critically ill patients with COVID-19 and the results were compared with patients with septic shock. The patients presented less requirement for vasoactive drugs, low plasma lactate levels, lower Interleukin 6 (IL6) levels, and greater sublingual microcirculatory flow than in septic patients. There was no difference in Syndecan-1 levels between both groups (endothelial activation).
When sublingual microcirculation was evaluated in COVID-19 patients using SDF Technology in different studies [8-11], they observed that this group of patients presented microcirculatory alterations with a pattern of increased capillary density, low percentage of perfused vessels, and high indices of heterogeneity. The observed capillary recruitment coincides with the findings of our study, where a high PPI evidences an increase in peripheral pulsatile flow.
A series of autopsies in COVID-19 patients compared with pathological anatomy of ARDS from other viral causes, observed in the lungs infected by SARS-CoV-2 distinctive vascular characteristics with severe endothelial injury with the presence of intracellular virus and microangiopathic thrombosis x 9 times higher than for Influenza (p < 0.001) and the number of neoformation vessels due to Intussusceptive angiogenesis is 2.7 times greater than in H1N1 (p < 0.001) [12,13]. These results are consistent with other studies showing angiocentric inflammation with large numbers of ACE2-positive endothelial cells in COVID-19 samples compared to healthy controls or Influenza “A” ARDS postmortem samples. This vascular injury is accompanied by thrombosis and intussusceptive angiogenesis in the lungs, heart, liver, kidney, brain, and lymphoreticular organs [14], organs with a high density of ACE2 receptors.
When DeBacker, et al. [24] evaluated sublingual microcirculation with Microscan imaging in patients with ARDS due to H1N1 and observed moderate to severe involvement with a sepsis-like pattern. The proportion of vessels perfused, and the microcirculatory flow index was negatively correlated with the SOFA score. The patients evaluated had high vasoactive requirements (norepinephrine 0.57 ± 0.55 ug/kg/min) and average lactate values of 1.8 mEq/L (1.2-3.7).
These microcirculatory findings, added to the differences described between the pathology of COVID-19 and H1N1 patients, show the different microcirculatory roles in the development of multiorgan dysfunction between these diseases, where the capillary recruitment component seems to have a central role in the pathophysiology of patients infected by SARS-CoV-2.
It is important to remember what we understand by capillary recruitment this is the increase in the number of perfused blood capillaries in response to different stimuli; either by opening existing capillaries through blood flow regulation mechanisms by arteriolar contraction or relaxation in response to increased metabolic demands or by the formation of new capillary vessels through “Angiogenesis” [25]. Those adaptive mechanisms were studied in muscles subjected to exercise [26]. Those works show that angiogenesis would be an adaptive response to the reduction in oxygen pressure, and or related to metabolic alterations mediated by the activation of different cytokines released by endothelial cells, such as Vascular endothelial growth factor (VEGF) activated by hypoxia; Fibroblast growth factor (FGF) in response to baroreceptor-mediated increased flow patterns generating shear forces in the vessel lumen wall; and (c) inflammatory mediators among others. These mechanisms could be involved in developing intussusceptive angiogenesis related to COVID-19 (findings in pathology), although it is unclear if similar angiogenesis mechanisms could occur in pathological states [27].
Reinforcing the concept that the target organ of the injury of this disease is the vascular endothelium, patients with respiratory failure due to SARS-CoV-2 show angiocentric inflammation with a high number of ACE2-positive endothelial cells compared with uninfected healthy controls and ARDS due to type “A” influenza [28]. In turn, it was evidenced that COVID-19 patients present high biomarkers of circulating endothelial stress, complement activation, and fibrinolytic dysregulation; and that these findings are associated with disease severity. This pattern of endothelial damage assessed by biomarkers differs from septic states [29].
Multiple studies describe the mechanisms involved by ACE2 receptors in both infection and damage mechanisms. The virus-receptor interaction reduces the action of ACE2, which produces an imbalance of the renin-angiotensin system (RAAS) and the kallikrein-kinin system (KKS). The decreased expression of ACE2 in endothelial cells fails to catalyze the conversion of angiotensin II to angiotensin, leading to the accumulation of angiotensin II, which is a potent autocrine, proinflammatory, and prothrombotic vasoconstrictor that would generate endothelial damage [30,31].
In turn, the decrease in ACE2 activity activates the KKS, which regulates many physiological processes such as inflammation, coagulation, vasodilation, and blood pressure.
Some authors [32] suggest that COVID-19 would produce “downregulation” of peptidases that inactivate bradykinins, substance P and neurotensin, increasing their vasodilator and proinflammatory activity and generating endothelial activation with increased vascular permeability. This process is called “Bradykinin Storm”. This increase in the levels of these vasoactive peptides is associated with an “upregulation” of bradykinin receptors (B1R and B2R) that would enhance their action. This postulated mechanism could be responsible for the increase in blood flow and loss of the hypoxic vasoconstrictor reflex in a COVID-19 patient, which would explain at least in part the presence of intrapulmonary shunt manifested in severe hypoxemia.
The exposed mechanisms triggered by endothelial activation and the observed pattern of angiogenesis-mediated capillary recruitment modifies the architecture of the microcirculatory bed by altering the rheological properties of blood flow (laminar/turbulent) generating blood flow heterogeneity with dispersed flow velocities, different forces of shearing on the vascular wall, segment of occluded vessels, platelet aggregation associated with up-regulation of thrombotic agonists.
These alterations in microcirculatory morphology could explain some of the findings of sublingual microcirculation studies in COVID-19 patients, such as the decrease in the percentage of perfused vessels, the microvascular flow index, and the increase in the heterogeneity index associated (convective component) with the recruitment capillary pattern manifested by increased capillary density (diffusive component) [8-10].
These microcirculatory disorders would be responsible for the different V/Q ratios at the lung level, which is one of the causes of the severe hypoxemia observed in COVID-19 patients. Severe hypoxemia with preserved lung mechanics in the early stages of the disease suggests that the intrapulmonary shunt would be due to vascular pathology [33,34].
Hypoxemia could also trigger and perpetuate this pattern of capillary recruitment previously explained by intrinsic autoregulation mechanisms of adaptive microvascular response to severe hypoxemia to improve tissue oxygenation, a mechanism studied in the hypoxia model due to height [35].
In our study, when we evaluated the relationship of PPI with the severity of hypoxemia according to the PaO2/FiO2 ratio, we did not observe significant variations (Figure 2), which would lead us to suspect that hypoxia would not be the main cause of capillary recruitment evaluated by PPI, perhaps having a role in the maintenance of the observed vascular disorder.
As in other studies, our patients presented arterial hypertension (HBP), diabetes (DBT), and obesity as the most frequent comorbidities. Well-known states of chronic endothelial injury with previously impaired capillarity make them more susceptible to endothelial activation and damage.
Finally, we found no relationship between PPI values and organ failure (Table 3). Elevated PPI values were not related to organ perfusion disorders. This, associated with low lactate levels, shows that microcirculation would not play a role in the organ failure associated with COVID-19 disease. The incidence of associated organic dysfunction would be explained by the high density of ACE2 receptors in the organs involved (lung, kidney, heart, liver) and therefore the greater susceptibility to direct viral injury and local endothelial alterations that would produce the microcirculatory changes described above, endothelial damage, thrombosis, and inflammation.
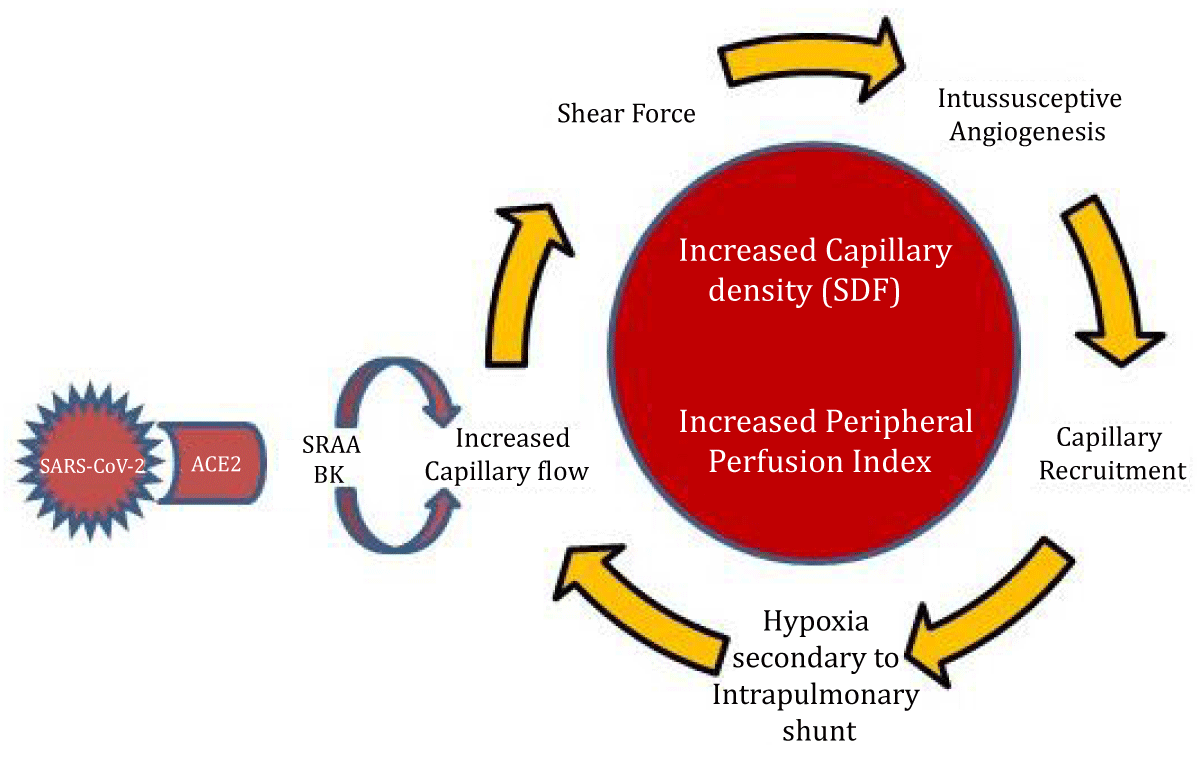
Summarizing, it could be stated that infection by SARS-CoV-2 via ACE2 receptors would produce an imbalance of vasoactive mediators (angiotensin/bradykinins) generating an increase in microcirculatory flow that via mechanoreceptors and release of endothelial cytokines would stimulate intussusceptive angiogenesis and loss of hypoxic vasoconstrictor reflex. All this associated with direct viral endothelial injury would produce endothelial activation, activating inflammation and the coagulation cascade. Altogether would generate the pattern of capillary recruitment observed in our study as high PPI and organ dysfunction due to local processes via ACE2 (Figure 3).
Figure 3: The SARS-CoV-2 interaction with ACE2 receptors would generate an imbalance of the Renin-Angiotensin and Bradykinin systems, producing a dysregulation of microcirculatory blood flow with a predominance of vasodilation with increased flow. The increase in microcirculatory flow produces a shear force on the capillary endothelium which, together with hypoxia, are strong stimuli of intussusceptive Angiogenesis responsible for the pattern of capillary recruitment. Hypoxia could be interpreted as an increase in the intrapulmonary shunt due to capillary recruitment/impaired hypoxic vasoconstrictor reflex and, in turn, stimulation of greater capillary recruitment. All these mechanisms together would result in a CAPILLARY RECRUITMENT Pattern that manifests as Increased Capillary Density in sublingual Microcirculation with SDF (Sidestream Dark Field) and Increased PPI.
Within the inflammatory parameters on admission, we observed a statistically significant greater increase in LDH in the patients who died. This tissue injury parameter was described by Cecconi, et al. as a predictor of poor outcomes in a cohort of COVID-19 patients [36]. A more recent work evaluated the levels of LDH isoenzymes in plasma in patients with SARS-CoV-2 infection. The high levels of LDH would not be due to LDH3 of pulmonary origin; consistent with our findings, the levels of LDH1 (heart, kidney, and erythrocytes) and LDH5 (liver and muscle) isozymes present in organs with high levels of ACE2 receptors [37].
The limitations of our study are due to its observational and descriptive nature, together with a small sample analyzed and some of the pathophysiological mechanisms exposed are purely speculative.
In our study of critically ill COVID-19, we found high PPI values, which could correspond to a pattern of capillary recruitment, and this phenomenon could not explain the associated organ damage. New studies with greater statistical power would be necessary to characterize the macro/microhemodynamic patterns, and their molecular correlates to correctly understand the development of multiple organ dysfunction in these patients.
Key messages
The peripheral perfusion index showed high values in a COVID-19 patient with associated multi-organ dysfunction.
The perfusion index showed a pattern of capillary recruitment, unable to demonstrate the cause of multiple organ dysfunctions by this mechanism.
The peripheral perfusion index did not show any relationship with the severity of hypoxemia.
The multi-organ dysfunction associated with COVID-19 could be explained by direct viral injury associated with ACE2 receptors.
There was no relationship between peripheral perfusion index values and outcome.
- Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020 Aug 21;24(1):516. doi: 10.1186/s13054-020-03240-7. PMID: 32825837; PMCID: PMC7441837.
- Loganathan S, Kuppusamy M, Wankhar W, Gurugubelli KR, Mahadevappa VH, Lepcha L, Choudhary AK. Angiotensin-converting enzyme 2 (ACE2): COVID 19 gate way to multiple organ failure syndromes. Respir Physiol Neurobiol. 2021 Jan;283:103548. doi: 10.1016/j.resp.2020.103548. Epub 2020 Sep 18. PMID: 32956843; PMCID: PMC7500408.
- Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and Multiorgan Response. Curr Probl Cardiol. 2020 Aug;45(8):100618. doi: 10.1016/j.cpcardiol.2020.100618. Epub 2020 Apr 28. PMID: 32439197; PMCID: PMC7187881.
- Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J Mol Histol. 2020 Dec;51(6):613-628. doi: 10.1007/s10735-020-09915-3. Epub 2020 Oct 4. PMID: 33011887; PMCID: PMC7533045.
- Loganathan S, Kuppusamy M, Wankhar W, Gurugubelli KR, Mahadevappa VH, Lepcha L, Choudhary AK. Angiotensin-converting enzyme 2 (ACE2): COVID 19 gate way to multiple organ failure syndromes. Respir Physiol Neurobiol. 2021 Jan;283:103548. doi: 10.1016/j.resp.2020.103548. Epub 2020 Sep 18. PMID: 32956843; PMCID: PMC7500408.
- De Backer D, Donadello K, Taccone FS, Ospina-Tascon G, Salgado D, Vincent JL. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care. 2011 Jul 19;1(1):27. doi: 10.1186/2110-5820-1-27. PMID: 21906380; PMCID: PMC3224481.
- Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM; Microcirculatory Alterations in Resuscitation and Shock Investigators. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007 Jan;49(1):88-98, 98.e1-2. doi: 10.1016/j.annemergmed.2006.08.021. Epub 2006 Nov 7. PMID: 17095120.
- Kanoore Edul VS, Caminos Eguillor JF, Ferrara G, Estenssoro E, Siles DSP, Cesio CE, Dubin A. Microcirculation alterations in severe COVID-19 pneumonia. J Crit Care. 2021 Feb;61:73-75. doi: 10.1016/j.jcrc.2020.10.002. Epub 2020 Oct 17. PMID: 33096349; PMCID: PMC7568145.
- Di Dedda U, Ascari A, Fantinato A, Fina D, Baryshnikova E, Ranucci M. Microcirculatory Alterations in Critically Ill Patients with COVID-19-Associated Acute Respiratory Distress Syndrome. J Clin Med. 2022 Feb 16;11(4):1032. doi: 10.3390/jcm11041032. PMID: 35207303; PMCID: PMC8876221.
- Abou-Arab O, Beyls C, Khalipha A, Guilbart M, Huette P, Malaquin S, Lecat B, Macq PY, Roger PA, Haye G, Bernasinski M, Besserve P, Soriot-Thomas S, Jounieaux V, Dupont H, Mahjoub Y. Microvascular flow alterations in critically ill COVID-19 patients: A prospective study. PLoS One. 2021 Feb 8;16(2):e0246636. doi: 10.1371/journal.pone.0246636. PMID: 33556081; PMCID: PMC7870020.
- Damiani E, Carsetti A, Casarotta E, Scorcella C, Domizi R, Adrario E, Donati A. Microvascular alterations in patients with SARS-COV-2 severe pneumonia. Ann Intensive Care. 2020 May 20;10(1):60. doi: 10.1186/s13613-020-00680-w. PMID: 32436075; PMCID: PMC7238400.
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417-1418. doi: 10.1016/S0140-6736(20)30937-5. Epub 2020 Apr 21. PMID: 32325026; PMCID: PMC7172722.
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020 Jul 9;383(2):120-128. doi: 10.1056/NEJMoa2015432. Epub 2020 May 21. PMID: 32437596; PMCID: PMC7412750.
- Jonigk D, Märkl B, Helms J. COVID-19: what the clinician should know about post-mortem findings. Intensive Care Med. 2021 Jan;47(1):86-89. doi: 10.1007/s00134-020-06302-0. Epub 2020 Nov 3. PMID: 33141245; PMCID: PMC7608308.
- Lima AP, Beelen P, Bakker J. Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Crit Care Med. 2002 Jun;30(6):1210-3. doi: 10.1097/00003246-200206000-00006. PMID: 12072670.
- Elshal MM, Hasanin AM, Mostafa M, Gamal RM. Plethysmographic Peripheral Perfusion Index: Could It Be a New Vital Sign? Front Med (Lausanne). 2021 Oct 1;8:651909. doi: 10.3389/fmed.2021.651909. PMID: 34660615; PMCID: PMC8517109.
- Rasmy I, Mohamed H, Nabil N, Abdalah S, Hasanin A, Eladawy A, Ahmed M, Mukhtar A. Evaluation of Perfusion Index as a Predictor of Vasopressor Requirement in Patients with Severe Sepsis. Shock. 2015 Dec;44(6):554-9. doi: 10.1097/SHK.0000000000000481. PMID: 26529657.
- van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020 Dec 14;24(1):696. doi: 10.1186/s13054-020-03400-9. PMID: 33317589; PMCID: PMC7735177.
- He H, Long Y, Liu D, Wang X, Zhou X. Clinical classification of tissue perfusion based on the central venous oxygen saturation and the peripheral perfusion index. Crit Care. 2015 Sep 14;19(1):330. doi: 10.1186/s13054-015-1057-8. PMID: 26369784; PMCID: PMC4568576.
- Korkut M, Bedel C, Selvi F, Zortuk O. Can peripheral perfusion index predict disease severity in COVID-19 patients in the emergency department? Ibnosina J Med Biomed Sci 2022; 14:35-40. Doi.org/10.1055/s-0042-1748776.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061-1069. doi: 10.1001/jama.2020.1585. Erratum in: JAMA. 2021 Mar 16;325(11):1113. PMID: 32031570; PMCID: PMC7042881.
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 May;8(5):475-481. doi: 10.1016/S2213-2600(20)30079-5. Epub 2020 Feb 24. Erratum in: Lancet Respir Med. 2020 Apr;8(4):e26. PMID: 32105632; PMCID: PMC7102538.
- Hutchings SD, Watchorn J, Trovato F, Napoli S, Mujib SF, Hopkins P, McPhail M. Microcirculatory, Endothelial, and Inflammatory Responses in Critically Ill Patients With COVID-19 Are Distinct From Those Seen in Septic Shock: A Case Control Study. Shock. 2021 Jun 1;55(6):752-758. doi: 10.1097/SHK.0000000000001672. PMID: 33021572.
- Salgado DR, Ortiz JA, Favory R, Creteur J, Vincent JL, De Backer D. Microcirculatory abnormalities in patients with severe influenza A (H1N1) infection. Can J Anaesth. 2010 Oct;57(10):940-6. doi: 10.1007/s12630-010-9365-6. Epub 2010 Jul 27. PMID: 20661679; PMCID: PMC7101965.
- Fry BC, Roy TK, Secomb TW. Capillary recruitment in a theoretical model for blood flow regulation in heterogeneous microvessel networks. Physiol Rep. 2013 Aug;1(3):e00050. doi: 10.1002/phy2.50. PMID: 24040516; PMCID: PMC3770315.
- Gustafsson T, Kraus WE. Exercise-induced angiogenesis-related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Front Biosci. 2001 Jan 1;6:D75-89. doi: 10.2741/gustafss. PMID: 11145922.
- Filipovic N, Tsuda A, Lee GS, Miele LF, Lin M, Konerding MA, Mentzer SJ. Computational flow dynamics in a geometric model of intussusceptive angiogenesis. Microvasc Res. 2009 Dec;78(3):286-93. doi: 10.1016/j.mvr.2009.08.004. Epub 2009 Aug 26. PMID: 19715707; PMCID: PMC2785026.
- Ackermann M, Mentzer SJ, Kolb M, Jonigk D. Inflammation and intussusceptive angiogenesis in COVID-19: everything in and out of flow. Eur Respir J. 2020 Nov 12;56(5):2003147. doi: 10.1183/13993003.03147-2020. PMID: 33008942; PMCID: PMC7530910.
- Fernández S, Moreno-Castaño AB, Palomo M, Martinez-Sanchez J, Torramadé-Moix S, Téllez A, Ventosa H, Seguí F, Escolar G, Carreras E, Nicolás JM, Richardson E, García-Bernal D, Carlo-Stella C, Moraleda JM, Richardson PG, Díaz-Ricart M, Castro P. Distinctive Biomarker Features in the Endotheliopathy of COVID-19 and Septic Syndromes. Shock. 2022 Jan 1;57(1):95-105. doi: 10.1097/SHK.0000000000001823. PMID: 34172614; PMCID: PMC8662948.
- Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020 Jul;251(3):228-248. doi: 10.1002/path.5471. Epub 2020 Jun 10. PMID: 32418199; PMCID: PMC7276767.`
- Rysz S, Al-Saadi J, Sjöström A, Farm M, Campoccia Jalde F, Plattén M, Eriksson H, Klein M, Vargas-Paris R, Nyrén S, Abdula G, Ouellette R, Granberg T, Jonsson Fagerlund M, Lundberg J. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat Commun. 2021 Apr 23;12(1):2417. doi: 10.1038/s41467-021-22713-z. PMID: 33893295; PMCID: PMC8065208.
- Karamyan VT. Between two storms, vasoactive peptides or bradykinin underlie severity of COVID-19? Physiol Rep. 2021 Mar;9(5):e14796. doi: 10.14814/phy2.14796. PMID: 33687143; PMCID: PMC7941673.
- Herrmann J, Mori V, Bates JHT, Suki B. Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat Commun. 2020 Sep 28;11(1):4883. doi: 10.1038/s41467-020-18672-6. PMID: 32985528; PMCID: PMC7522238.
- Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020 May 15;201(10):1299-1300. doi: 10.1164/rccm.202003-0817LE. PMID: 32228035; PMCID: PMC7233352.
- Hilty MP, Merz TM, Hefti U, Ince C, Maggiorini M, Pichler Hefti J. Recruitment of non-perfused sublingual capillaries increases microcirculatory oxygen extraction capacity throughout ascent to 7126 m. J Physiol. 2019 May;597(10):2623-2638. doi: 10.1113/JP277590. Epub 2019 Mar 28. PMID: 30843200; PMCID: PMC6826230.
- Cecconi M, Piovani D, Brunetta E, Aghemo A, Greco M, Ciccarelli M, Angelini C, Voza A, Omodei P, Vespa E, Pugliese N, Parigi TL, Folci M, Danese S, Bonovas S. Early Predictors of Clinical Deterioration in a Cohort of 239 Patients Hospitalized for Covid-19 Infection in Lombardy, Italy. J Clin Med. 2020 May 20;9(5):1548. doi: 10.3390/jcm9051548. PMID: 32443899; PMCID: PMC7290833.
- Serrano-Lorenzo P, Coya ON, López-Jimenez A, Blázquez A, Delmiro A, Lucia A, Arenas J, Martín MA; COVID-19 ’12 Octubre’ Hospital Clinical Biochemistry Study Group. Plasma LDH: A specific biomarker for lung affectation in COVID-19? Pract Lab Med. 2021 May;25:e00226. doi: 10.1016/j.plabm.2021.e00226. Epub 2021 Apr 21. PMID: 33898686; PMCID: PMC8058053.